Effects of Acid Rain
Enroll to start learning
You’ve not yet enrolled in this course. Please enroll for free to listen to audio lessons, classroom podcasts and take practice test.
Interactive Audio Lesson
Listen to a student-teacher conversation explaining the topic in a relatable way.
Introduction to Acid Rain
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Acid rain occurs when sulfur and nitrogen oxides are emitted into the atmosphere from industrial activities and fossil fuel combustion. Can anyone tell me what these oxides form in the atmosphere?

They form acids like sulfuric acid and nitric acid.

Exactly right! These acids lower the pH of rainwater, causing it to become acidic. What is the pH level at which rainwater is considered acidic?

When it falls below 5.6.

Great! So, if clean rain is around 5.6, what happens to rain with a pH lower than that?

It becomes harmful to the environment.

That's right! Acid rain can lead to several adverse effects.
Effects on Infrastructure
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Can someone think of an example of a structure affected by acid rain?

The Taj Mahal!

Correct! Acid rain causes deterioration of marble buildings. What type of crystals forms as a result of this corrosion?

Calcium and magnesium sulfate.

Exactly! How does this affect historical monuments?

It leads to them being damaged or eroded over time.

Great insights! It's essential to protect these structures from further damage, isn't it?
Impact on Aquatic Life
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Let's discuss how acid rain affects aquatic ecosystems. Any thoughts on how fish populations are affected?

The lakes become acidic and can kill fish.

Right! What happens to fish when the water pH drops significantly?

They might experience reproductive issues and even die.

Exactly! Acid rain can lead to lakes becoming fishless, especially in regions like Sweden and Norway. Why do you think this is a critical issue?

Because it affects the whole ecosystem.

Exactly! Healthy aquatic life is vital for a balanced ecosystem.
Damage to Vegetation
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

How does acid rain affect trees?

It weakens them and makes them vulnerable.

That's spot on! Acid rain can damage foliage and make trees susceptible to disease. Can anyone explain why this is particularly problematic?

If trees are weak, they can't survive harsh conditions.

Exactly! Conditions like extreme temperatures or drought become much harder for stressed trees!
Control Measures
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

What proactive measures can we take to control acid rain?

Reducing emissions from industries.

That's right! What else can we do to correct the impact already caused by acid rain?

Liming lakes can help neutralize acidity.

Great! Liming is effective in restoring pH balance. What about the use of protective measures for infrastructure?

Using coatings to protect buildings?

Exactly! Protecting our buildings can extend their lifespan.
Introduction & Overview
Read summaries of the section's main ideas at different levels of detail.
Quick Overview
Standard
Acid rain is caused by the oxidation of sulfur and nitrogen oxides from industrial activity and fossil fuel combustion, leading to the formation of nitric and sulfuric acids that have detrimental effects on infrastructure, aquatic life, and ecosystems at large. Measures to reduce its impact include emission controls and liming of affected areas.
Detailed
Effects of Acid Rain
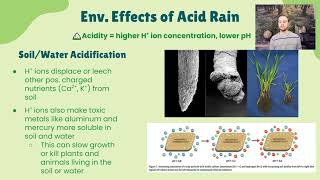
Acid rain stems from the oxidation of sulfur and nitrogen oxides generated from industrial operations and the burning of fossil fuels. When these gases are released into the atmosphere, they are transported over long distances, ultimately converting into sulfuric and nitric acids. Rain becomes acidic when its pH falls below 5.6, with normal rainwater having a pH of about 5.6 due to dissolved carbon dioxide forming carbonic acid.
Key Effects:
- Infrastructure Deterioration: Acid rain significantly damages buildings, especially those made of marble, by causing corrosion. Notable monuments like the Taj Mahal have suffered from this effect.
- Damage to Statues: Many historical stone statues in regions like Greece and Italy are partially dissolved due to acid rain.
- Impact on Aquatic Life: Fish and other aquatic organisms are severely affected because acidic lakes can lead to reproductive failures and even fish deaths. Many lakes in Sweden, Norway, and Canada have become fishless.
- Soil and Vegetation: Acid rain weakens trees and makes them more vulnerable to diseases, pests, and environmental stresses.
Control Measures:
- Reduce emissions of SO2 and NOx through pollution control equipment.
- Use liming to neutralize acidic soils and lakes.
- Apply protective polymer coatings in water supply systems.
Youtube Videos








Key Concepts
-
Acid Rain: Resulting from the oxides of sulfur and nitrogen, it causes harm to the environment.
-
pH Level: Indicates the acidity of rain; below 5.6 is a concern.
-
Deterioration of Infrastructure: Acid rain can corrode buildings and monuments.
-
Impact on Aquatic Life: Acid rain negatively affects fish and other aquatic systems.
-
Vegetation Damage: Acid rain weakens trees making them susceptible to disease.
Examples & Applications
The Taj Mahal is experiencing ongoing deterioration due to acid rain damage.
Many lakes in Sweden have become fishless due to the lethal effects of acid rain on aquatic life.
Memory Aids
Interactive tools to help you remember key concepts
Rhymes
Acid rain, oh what a pain, it wears away the stone and grain.
Stories
Once, a magnificent monument stood tall, until acid rain began to call. It wept and cried as its beauty broke, the weight of pollution became the joke.
Memory Tools
Remember your COLD WATER: C- Control emissions; O- Offer liming; L- Let tanks coat; D- Deterioration managed; W- Watch pH; A- Aquatic health saved; T- Trees protected; E- Effects studied; R- Renew.
Acronyms
SAFE
S- Sulfur & Nitrogen oxides; A- Acidic rain; F- Fish populations hurt; E- Ecosystems damaged.
Flash Cards
Glossary
- Acid Rain
Rain with a pH of less than 5.6, primarily caused by sulfuric and nitric acid from atmospheric pollutants.
- pH
A measure of how acidic or basic a solution is, with lower values indicating increased acidity.
- Sulfur Dioxide (SO2)
A colorless gas that is a significant contributor to acid rain when oxidized in the atmosphere.
- Nitrogen Oxides (NOx)
Gases resulting from the combustion of fossil fuels which contribute to acid rain.
- Liming
The process of adding lime (calcium carbonate) to acidic soils or water bodies to neutralize acidity.
Reference links
Supplementary resources to enhance your learning experience.
