Factors Affecting Evaporation
Enroll to start learning
You’ve not yet enrolled in this course. Please enroll for free to listen to audio lessons, classroom podcasts and take practice test.
Interactive Audio Lesson
Listen to a student-teacher conversation explaining the topic in a relatable way.
Solar Radiation
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Today, let's begin with the role of solar radiation in evaporation. Can anyone tell me what solar radiation is?

It's the energy we get from the sun!

Correct! Solar radiation is the primary energy source for evaporation. The more solar energy available, the more water can evaporate.

So, does that mean on a cloudy day, evaporation is reduced?

Exactly! Clouds limit solar radiation, leading to lower evaporation rates. Remember, more sun equals more evaporation! Let’s summarize: solar radiation is directly proportional to the evaporation rate.
Temperature
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Moving on to temperature—how does temperature affect evaporation rates?

Higher temperatures increase evaporation, right?

Absolutely! Higher air and water temperatures speed up the evaporation process because warm air can hold more moisture, thus enhancing the vapor pressure gradient. Can anyone think of an example?

I guess, in the summer, water in a lake evaporates faster.

Precisely! Great observation. Remember: higher temperatures lead to faster evaporation.
Humidity
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Let’s discuss humidity. Who can explain its effect on evaporation?

Higher humidity makes evaporation slower because the air is already full of moisture.

Exactly! High relative humidity decreases the vapor pressure gradient, which is crucial for evaporation. Can someone explain why?

If the air is saturated, there’s less room for water vapor to escape.

Fantastic! So remember, high humidity = less evaporation. It’s inversely related.
Wind Speed
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Now, let’s talk about wind speed. How does wind help in evaporation?

Wind blows away the moist air on the water's surface.

Right! Wind removes the saturated layer of air and keeps the vapor pressure gradient high, significantly enhancing evaporation rates.

So is that why it evaporates faster in open areas compared to sheltered ones?

Absolutely! Wind speed directly increases evaporation. Keep that in mind: think of wind as a supporter of evaporation!
Atmospheric Pressure and Water Quality
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Finally, let’s discuss atmospheric pressure and water quality. Who remembers how atmospheric pressure affects evaporation?

Lower pressure increases evaporation because it lowers the boiling point.

Correct! Lower atmospheric pressure facilitates evaporation. Now, how does water quality fit in here?

Impurities slow down evaporation because they lower the vapor pressure!

Excellent! Water quality, particularly salinity, affects how easily water can evaporate. Remember these two factors—lower pressure and clean water mean higher evaporation!
Introduction & Overview
Read summaries of the section's main ideas at different levels of detail.
Quick Overview
Standard
Factors such as solar radiation, temperature, humidity, wind speed, and atmospheric pressure play significant roles in determining the rate of evaporation. Each factor influences the evaporation process in distinct ways, affecting water loss and management in hydrology, agriculture, and environmental studies.
Detailed
Factors Affecting Evaporation
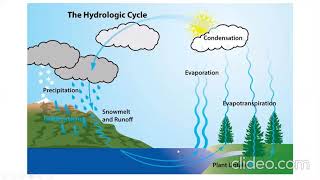
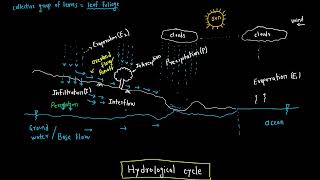
Evaporation, a key process in the hydrological cycle, is influenced by a variety of factors that can affect its rate significantly. Understanding these factors is crucial for water resource management and agricultural planning. Below are some of the main factors affecting evaporation:
1. Solar Radiation
- Primary Source: Solar radiation is the main energy source for evaporation.
- Direct Proportionality: The rate of evaporation increases with greater solar radiation, which enhances the energy available for water to convert into vapor.
2. Temperature
- Heat Acceleration: Higher temperatures of air and water increase the rate of evaporation since warm air can hold more moisture, creating a steeper gradient for vapor movement.
3. Humidity
- Inverse Relationship: Higher humidity levels lower the gradient for evaporation. High relative humidity reduces the vapor pressure gradient between the water surface and the air, thereby inhibiting evaporation.
4. Wind Speed
- Enhancement of Evaporation: Wind effectively removes the saturated layer of air from the water surface, maintaining a higher vapor pressure gradient, which significantly increases the evacuation rate in open water bodies.
5. Atmospheric Pressure
- Low Pressure Effects: Lower atmospheric pressure reduces the boiling point of water, consequently increasing evaporation rates as it allows easier transition from liquid to vapor.
6. Water Quality
- Impacts of Impurities: The presence of impurities and salts in water can affect the evaporation rate. For example, saline water tends to evaporate more slowly due to a reduced vapor pressure.
7. Surface Area
- Exposed Area Contribution: A larger surface area exposed to the air can significantly increase the total evaporation because more water molecules are available to escape into the vapor phase.
Youtube Videos







Audio Book
Dive deep into the subject with an immersive audiobook experience.
Solar Radiation
Chapter 1 of 7
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Solar Radiation
- Primary energy source for evaporation.
- Directly proportional to evaporation rate.
- Greater solar radiation increases the energy available for converting water to vapor.
Detailed Explanation
Solar radiation is the main source of energy that drives the process of evaporation. When the Sun shines on water, it provides the energy needed for the water molecules to break free from the liquid state and turn into vapor. The more solar radiation there is, the higher the rate of evaporation. This means that on sunny days, when there is a lot of sunlight, we can expect more water to evaporate compared to cloudy days.
Examples & Analogies
Think of solar radiation as a heater for a pot of water. The more heat you apply, the faster the water boils and turns into steam. Similarly, on a sunny day, the 'heat' from the sun warms the water, and more of it evaporates into vapor.
Temperature
Chapter 2 of 7
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Temperature
- Higher air and water temperatures accelerate evaporation.
- Warm air holds more moisture, enhancing the gradient for vapor movement.
Detailed Explanation
Temperature plays a crucial role in evaporation as it affects how much energy water molecules have. When either the air or the water temperature increases, the water molecules move faster, making it easier for them to escape into the vapor phase. Additionally, warmer air has a higher capacity to hold moisture, creating a bigger difference (or gradient) between the moisture content of the air and the water surface. This encourages even more evaporation.
Examples & Analogies
Consider how a warm bath feels compared to a lukewarm one. You might notice that warm water evaporates quicker and feels steamy. This is similar to how the temperature increases the speed of evaporation in nature.
Humidity
Chapter 3 of 7
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Humidity
- Inversely related to evaporation.
- High relative humidity reduces the vapor pressure gradient between water surface and air.
Detailed Explanation
Humidity refers to the amount of moisture present in the air. When humidity is high, the air is already filled with water vapor, which makes it harder for additional water molecules to evaporate. This creates a condition where the evaporative drive (the difference between the vapor pressure of water and the air) is reduced. Therefore, evaporation slows down when humidity levels are high.
Examples & Analogies
Think of a crowded room filled with people. It becomes harder for new people to come in because the space is already occupied. Similarly, when the air is humid, it's 'crowded' with vapor, making it difficult for more water molecules to escape.
Wind Speed
Chapter 4 of 7
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Wind Speed
- Wind removes the saturated air layer near the water surface, maintaining a high vapor pressure gradient.
- Increases evaporation rate significantly in open water bodies.
Detailed Explanation
Wind plays an important role in evaporation by moving away the saturated air that forms over the surface of the water. When the air near the water’s surface becomes saturated with moisture, it reduces the capacity for further evaporation. Wind helps by replacing this moist air with drier air, which maintains a steep vapor pressure gradient and promotes higher rates of evaporation.
Examples & Analogies
Imagine a sponge that is soaked with water. If you place it in a moving breeze, the airflow helps the water escape faster than if it were just sitting still. This is similar to how wind increases evaporation rates.
Atmospheric Pressure
Chapter 5 of 7
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Atmospheric Pressure
- Lower atmospheric pressure reduces the boiling point of water, thereby increasing evaporation.
Detailed Explanation
Atmospheric pressure refers to the weight of the air above us. When atmospheric pressure is lower, as it is at higher altitudes, the boiling point of water decreases. This means that water can evaporate more easily because it doesn’t require as much heat to change from liquid to vapor. Thus, at lower pressures, evaporation occurs at a faster rate.
Examples & Analogies
If you've ever boiled water at high altitude, like on a mountain, you may have noticed that it starts to boil at a lower temperature than it does at sea level. This reduction in boiling point is similar to how lower pressure helps water evaporate faster.
Water Quality
Chapter 6 of 7
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Water Quality
- Impurities and dissolved salts can alter evaporation rates.
- Saline water evaporates slower due to reduced vapor pressure.
Detailed Explanation
The quality of the water can significantly affect evaporation rates. Pure water will evaporate more quickly than water that contains impurities or dissolved salts. When salts are dissolved in the water, they lower the vapor pressure of the water, meaning fewer water molecules can escape into the air. This results in a slower rate of evaporation.
Examples & Analogies
Imagine trying to boil a pot of pure water versus one with a lot of salt in it. The salty water will take longer to boil and evaporate. This is because the salt interferes with the water molecules’ ability to escape as vapor.
Surface Area
Chapter 7 of 7
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Surface Area
- A larger exposed surface area leads to higher total evaporation.
Detailed Explanation
The surface area of the water body directly impacts the rate of evaporation. The larger the surface area exposed to the air, the more water molecules are available to escape into the vapor phase. This is why evaporation rates increase with larger lakes or ponds compared to smaller containers of water.
Examples & Analogies
Think about a large swimming pool versus a small cup of water. The pool has much more water surface exposed to the air, and will thus lose a lot more water to evaporation compared to the small cup, which has a limited surface area.
Key Concepts
-
Solar Radiation: The main energy source for evaporation.
-
Temperature: Increased temperatures enhance evaporation rates.
-
Humidity: High humidity levels inversely affect evaporation.
-
Wind Speed: Wind enhances evaporation by removing saturated air.
-
Atmospheric Pressure: Lower pressure increases evaporation rates.
-
Water Quality: Impurities in water can slow evaporation.
-
Surface Area: Larger surface areas increase total evaporation.
Examples & Applications
In summer, lakes can lose more water due to high temperatures and solar radiation.
A windy day at the beach leads to faster evaporation of water compared to a calm day.
Memory Aids
Interactive tools to help you remember key concepts
Rhymes
Heat and light from the sun's bright rays, make water vaporize in sunny days.
Stories
Once, in a land where sun shined bright, water evaporated in joyful flight, but when the air was humid and full, the water stayed, and the air was dull.
Memory Tools
Use the acronym SHAW (Solar radiation, Humidity, Air pressure, Wind speed) to remember key evaporation factors.
Acronyms
Think of the word 'THAW' (Temperature, Humidity, Air pressure, Wind) - these are the factors that can cause evaporation to increase.
Flash Cards
Glossary
- Evaporation
The process by which water changes from liquid to vapor due to energy absorption.
- Solar Radiation
Energy emitted by the sun, crucial as the primary source for evaporation.
- Temperature
A measure of the warmth or coldness of the environment, affects evaporation rates.
- Humidity
The amount of water vapor present in the air, inversely related to evaporation.
- Wind Speed
The speed at which air moves; higher wind speeds enhance evaporation.
- Atmospheric Pressure
The pressure exerted by the weight of air above; affects boiling point and evaporation.
- Water Quality
The chemical, physical, and biological characteristics of water, impacting evaporation rate.
- Surface Area
The total area exposed to the air; larger areas promote higher evaporation.
Reference links
Supplementary resources to enhance your learning experience.
