Functional Groups and Nomenclature
Enroll to start learning
You’ve not yet enrolled in this course. Please enroll for free to listen to audio lessons, classroom podcasts and take practice test.
Interactive Audio Lesson
Listen to a student-teacher conversation explaining the topic in a relatable way.
Introduction to Functional Groups
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Today, we're going to start discussing functional groups, which are specific groups of atoms within molecules that dictate how those molecules behave chemically. Can anyone tell me why functional groups are important?

They help us predict how a molecule will react?

Exactly! Different functional groups have unique properties and reactivity patterns. For example, alcohols (-OH) and carboxylic acids (-COOH) behave very differently in reactions. We'll go over a few common functional groups today.

What are some examples of those functional groups?

Good question! Some examples include alkanes, alkenes, and ketones. Let's remember them using the acronym AAK: Alkanes, Alkenes, and Ketones. Repeat after me: AAK!

AAK!

Fantastic! So what is the main functional group of alkanes?

C–C single bonds.

Correct! Alkanes are saturated hydrocarbons with the general formula CnH2n+2. Who can explain the significance of saturation here?

It means that they only have single bonds and can’t react to form more bonds.

Exactly! Saturated hydrocarbons don't have double or triple bonds, which makes them less reactive. Let's recap: functional groups affect reactivity, and the first group we covered is alkanes, which are saturated hydrocarbons.
Common Functional Groups
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Now let's dive deeper into common functional groups. Who can tell me the functional group associated with alkenes?

C=C double bonds!

Correct! Alkenes are unsaturated hydrocarbons. Their general formula is CnH2n. What's an example of a reaction they might undergo?

They can undergo electrophilic addition.

Exactly! Let's visualize this: alkenes can react quickly because of their double bonds. Now what about alkynes? Any guesses?

They have triple bonds, right?

That's right! Their general formula is CnH2n-2. Alkenes and alkynes are more reactive than alkanes because of their multiple bonds. Remember our acronym AAK, and we'll add another A for Alkynes!

AAKA!

Great! Lastly, how do we recognize functional groups in our nomenclature?

By identifying the longest carbon chain and its substituents.

Exactly! Nomenclature is all about following the steps systematically. Keep practicing these groups, and you'll see how they influence compound reactivity!
IUPAC Nomenclature Rules
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Now that we understand functional groups, let’s focus on naming organic compounds using IUPAC nomenclature. What is the first step?

Identify the longest carbon chain.

Exactly! This gives us the base name. What comes next after identifying the longest chain?

Number the chain so that the highest-priority functional group has the lowest locant.

Correct! High priority groups determine the suffix of the name. Can anyone explain why the position matters?

To make sure we can precisely communicate which functional groups are where!

Absolutely! Next is identifying and naming substituents. What do we do with them?

List them alphabetically even if they come from the same group.

Exactly right! Let’s try an example. If we have three carbons in the main chain and a methyl group at carbon 2, which is how we would name it?

It would be 2-methylpropane.

Well done! We covered some key IUPAC naming details today. Remember the steps, and you'll become proficient in organic nomenclature.
Introduction & Overview
Read summaries of the section's main ideas at different levels of detail.
Quick Overview
Standard
In this section, we explore various functional groups that determine the reactivity of organic compounds and how to systematically name them using IUPAC nomenclature. Understanding these concepts is crucial for predicting chemical behavior and communicating the structures of organic molecules effectively.
Detailed
Detailed Summary
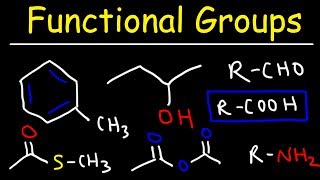
Functional groups are defined as specific groups of atoms within organic compounds that determine their chemical reactivity and properties. Recognizing these groups is critical for understanding organic chemistry. This section introduces various functional groups, including alkanes, alkenes, alkynes, aromatic groups, alcohols, and more, detailing their general formulas, naming strategies, and chemical behaviors.
The systematic naming of organic compounds follows the guidelines established by the International Union of Pure and Applied Chemistry (IUPAC). This involves several steps: identifying the longest carbon chain, numbering it to assign the lowest locants to functional groups, and naming any substituents alphabetically. Special techniques for naming compounds with multiple functional groups and stereochemistry are also discussed.
By mastering the recognition of functional groups and IUPAC nomenclature, students will gain the skills necessary to analyze organic compounds, predict their reactivity, and communicate chemical structures effectively.
Youtube Videos


Audio Book
Dive deep into the subject with an immersive audiobook experience.
Importance of Functional Groups
Chapter 1 of 5
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Functional groups are specific arrangements of atoms within molecules that are responsible for characteristic chemical reactions. Recognizing functional groups is the first step in understanding reactivity and naming organic molecules systematically according to IUPAC rules.
Detailed Explanation
Functional groups are groups of atoms that determine the chemical reactions of a molecule. For example, a hydroxyl group (-OH) in alcohols makes them behave differently than hydrocarbons that do not have this group. By identifying these functional groups, chemists can predict how a compound will react in different situations. The IUPAC rules help in naming these compounds appropriately, making communication about them clearer among scientists.
Examples & Analogies
Think of functional groups as the flavoring of a dish. Just like how adding salt or spices can change the taste of food, adding different functional groups alters how molecules behave in a chemical reaction. If you know a dish contains garlic (like the presence of a specific functional group), you can predict its taste without tasting it.
Common Functional Groups Overview
Chapter 2 of 5
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Below is a non-exhaustive list of widely encountered functional groups, their general formulas, and brief reactivity notes. Each is discussed in turn with examples and naming guidelines.
Detailed Explanation
This section provides a list of various functional groups that chemists frequently encounter. Each group has a specific formula and property associated with it, which influences the type of reactions they participate in. For instance, alkanes are saturated hydrocarbons characterized by C–C single bonds and generally have low reactivity, whereas alkenes contain C=C double bonds and are more reactive due to their electron-rich nature. Understanding these characteristics helps in predicting how these compounds will interact with other chemicals.
Examples & Analogies
Imagine a toolbox where each tool can perform different tasks. Functional groups are like the different tools in that toolbox—each one serves a unique role in helping scientists understand how various compounds can be built and how they will behave chemically.
Alkanes - Saturated Hydrocarbons
Chapter 3 of 5
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
- Alkanes (Saturated Hydrocarbons)
- Formula: CnH2n+2 (for open chains)
- Functional group: C–C single bonds only (all carbons are sp3-hybridized)
- Naming: Root name based on the longest carbon chain (meth-, eth-, prop-, but-, pent-, hex-, hept-, oct-, non-, dec-, etc.) plus “-ane.” Any substituents (alkyl groups) named alphabetically with locants indicating position.
- Example: CH3–CH2–CH2–CH3 is butane.
- Example of branching: (CH3)2CH–CH2–CH3 is named 2-methylpentane (longest chain five carbons, methyl substituent on carbon 2).
- Reactivity: Generally inert to many reagents; undergoes free-radical halogenation (in presence of light or radical initiator), combustion, cracking under strong conditions.
Detailed Explanation
Alkanes are the simplest type of hydrocarbons, containing only single bonds between carbon atoms. Their formula follows a specific pattern—CnH2n+2—indicating how hydrogens increase as carbons are added. Alkanes are named based on the longest chain of carbons, and branches are noted with locants to describe their positions. They are generally stable and do not react with many substances, but can be transformed under specific conditions, like being halogenated or burned. This stability is important in many industrial applications where reactions need to be controlled.
Examples & Analogies
Think of alkanes as a simple Lego structure made of blocks (carbons). Each block (carbon) is held together by strong connections (single bonds), creating a stable shape (the molecule) that doesn’t easily change or fall apart, unless you add in some special pieces (like heat or light, which can cause the structure to react).
Alkenes - Carbon-Carbon Double Bonds
Chapter 4 of 5
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
- Alkenes (Carbon–Carbon Double Bonds)
- Functional group: C=C (sp2-hybridized carbons), allowing planar geometry at the double bond.
- Naming: Identify the longest chain containing the C=C double bond; use suffix “-ene.” Number the chain so that the double bond carbon has the lowest possible locant. For multiple double bonds, use “-diene,” “-triene,” etc., with appropriate locants. For cyclic alkenes, prefix “cyclo-” and name as “cycloalkene.”
- Example: CH2=CH–CH2–CH3 is 1-butene (double bond between C1 and C2).
- Example: CH3–CH=CH–CH3 is 2-butene. If substituents exist, specify E/Z stereochemistry: trans-2-butene is (E)-2-butene, cis-2-butene is (Z)-2-butene.
- Reactivity: Undergoes electrophilic addition (e.g., HBr addition, hydration, halogenation), polymerization (in presence of catalysts like Ziegler–Natta), and oxidation (e.g., ozonolysis cleaves double bond).
Detailed Explanation
Alkenes are hydrocarbons characterized by at least one carbon-carbon double bond (C=C), making them more reactive than alkanes. Their ability to form double bonds allows them to undergo several types of reactions, including addition reactions where new atoms are added to the double bond. Naming rules require defining the longest chain that includes the double bond, and it is given the suffix '-ene'. These reactions and rules help predict how alkenes behave in chemical processes.
Examples & Analogies
Think of alkenes like a tightly wound rubber band (the double bond) in a tug-of-war: it has a certain tension that can be released (reactivity) when pulled in the right way (during a reaction). When you apply force correctly, the rubber band can snap and change shape (react and form new products).
Alcohols - Hydroxyl Functional Group
Chapter 5 of 5
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
- Alcohols (–OH)
- General formula: R–OH, where R is an alkyl or aryl group. The oxygen is sp3-hybridized, bearing two lone pairs and forming polar O–H and C–O bonds.
- Classification:
- Primary (1°) alcohol: R–CH2–OH (hydroxyl-bearing carbon is bonded to one other carbon).
- Secondary (2°) alcohol: R–CH(OH)–R′ (carbon bonded to two other carbons).
- Tertiary (3°) alcohol: R–C(OH)(R′)(R″) (carbon bonded to three other carbons).
- Naming: Identify the longest carbon chain containing the –OH group, replace “-e” of alkane with “-ol,” and number so that –OH carbon has lowest possible locant. For multiple –OH groups, use “-diol,” “-triol,” etc., with appropriate locants. If the compound has priority functional groups (e.g., carboxylic acid and alcohol), use “hydroxy-” as a prefix rather than “-ol.”
- Physical properties: Hydrogen bonding leads to higher boiling points compared to hydrocarbons of similar molar mass. Solubility in water depends on chain length: short-chain alcohols (methanol, ethanol, 1-propanol) are miscible with water, whereas longer chains become progressively less soluble.
- Reactivity:
- Can be protonated on oxygen, making OH a good leaving group (as water) under acid catalysis.
- React with acids to form esters (esterification).
- Undergo oxidation: primary alcohols oxidize to aldehydes (mild) and further to carboxylic acids (strong), secondary alcohols oxidize to ketones, tertiary alcohols typically resist oxidation unless under strenuous conditions (leading to C–C cleavage).
Detailed Explanation
Alcohols are characterized by the hydroxyl group (-OH) and have unique properties because of hydrogen bonding. They are classified based on the number of carbons attached to the carbon bearing the hydroxyl, which influences their reactivity and boiling points. Alcohols can react with acids to form esters and can also be oxidized to form aldehydes or ketones, depending on their classification. This versatility is used in many chemical processes and applications.
Examples & Analogies
Consider alcohols as tall trees with branches. The -OH group represents the trunk, while the branches signify how many carbons surround it. The more branches (carbons) the tree has, the more stable it is (less reactive), and just like trees produce more fruit (different chemical products) in season, alcohols can convert through reactions to form other compounds.
Key Concepts
-
Functional Groups: Specific groups of atoms determining reactivity.
-
Alkanes: Saturated hydrocarbons with single C–C bonds.
-
Alkenes: Unsaturated hydrocarbons with C=C double bonds.
-
Alkynes: Unsaturated hydrocarbons with C≡C triple bonds.
-
IUPAC Nomenclature: Systematic naming of organic compounds.
Examples & Applications
Butane (C4H10) is an example of an alkane.
Ethene (C2H4) demonstrates the characteristics of an alkene.
Propyne (C3H4) is an example of an alkyne.
Memory Aids
Interactive tools to help you remember key concepts
Rhymes
Alkanes, alkenes, and alkynes three, keep them straight, that's the key!
Stories
Imagine a family: Alkas, the alkanes, are happy and saturated, while their siblings, Alkies, the alkenes and alkynes, can't wait to react and mix.
Memory Tools
AAKA - Alkanes, Alkenes, Ketones, Alkynes is a memorable order for functional groups.
Acronyms
Use the acronym FLAME for functional groups
Functional
Longest chain
Alkyl groups
Multiple bonds
and Ending suffix.
Flash Cards
Glossary
- Functional Group
Specific arrangement of atoms in a molecule responsible for its characteristic reactions.
- Alkane
Saturated hydrocarbons with only single C–C bonds.
- Alkene
Unsaturated hydrocarbons with at least one C=C double bond.
- Alkyne
Unsaturated hydrocarbons with at least one C≡C triple bond.
- IUPAC Nomenclature
Systematic naming of organic compounds using rules established by the International Union of Pure and Applied Chemistry.
Reference links
Supplementary resources to enhance your learning experience.
