Polymers and Their Applications
Enroll to start learning
You’ve not yet enrolled in this course. Please enroll for free to listen to audio lessons, classroom podcasts and take practice test.
Interactive Audio Lesson
Listen to a student-teacher conversation explaining the topic in a relatable way.
Classification of Polymers
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Today we start by discussing the classification of polymers. Can anyone tell me what a polymer is?

A polymer is a large molecule made up of repeating units called monomers!

Exactly! Now, polymers can be classified into two categories: natural and synthetic. Who can give examples of each?

Natural polymers include things like proteins and starches, while synthetic ones include plastics like polyethylene.

Great job! Remember the acronym 'NAPS' for Natural — Amino acids (proteins), Polysaccharides (like starch), or Synthetic materials like PE and PVC. What does this classification tell us about their usage?

Natural polymers are often biodegradable, while synthetic polymers are commonly used in industrial applications because they can be engineered for specific properties.

Absolutely! That's a key point. In summary, natural polymers are biologically sourced, and synthetic polymers are human-made.
Thermoplastics vs. Thermosets
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Next, let's discuss the difference between thermoplastics and thermosets. Who can explain what thermoplastics are?

Thermoplastics are polymers that can be melted and reshaped. They become soft with heat and can be reshaped multiple times.

Correct! Now, what about thermosets?

Thermosets cannot be remolded once they are set. They are permanently hardened.

Exactly! So remember, 'Melt or Never' — is a helpful way to remember: Thermoplastics melt, thermosets never melt. Can anyone name some examples of each?

For thermoplastics, we have polyethylene and PVC. For thermosets, there’s epoxy and vulcanized rubber.

Well done! In conclusion, thermoplastics are flexible and reusable, whereas thermosets are rigid and permanent.
Mechanisms of Polymerization
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Now, let's explore how different types of polymerization occur. What is one method of polymerization?

Radical polymerization is one method where radicals initiate the polymer chain.

Correct! Can someone explain the three stages of radical polymerization?

There’s initiation, propagation, and termination.

Exactly! Initiation starts the chain, propagation builds it, and termination ends the reaction. Remember: 'I Put Tasty snacks in lunch.' Can anyone name another type of polymerization?

Ionic polymerization, right?

Yes! Ionic polymerization can be cationic or anionic. In conclusion, this understanding of polymerization processes is crucial because it helps in designing polymers for specific applications.
Introduction & Overview
Read summaries of the section's main ideas at different levels of detail.
Quick Overview
Standard
This section outlines the classification of polymers into natural and synthetic types, thermoplastics and thermosets, addition versus condensation polymers, and explores various polymerization mechanisms and their applications in industries such as textiles, automotive, and biomedical fields.
Detailed
Polymers and Their Applications
Polymers are significant macromolecules that consist of repeating units, known as monomers. These substances play a crucial role in a variety of applications in our daily lives, from packaging materials and textiles to adhesives and biological systems. They can be broadly categorized based on their source (natural vs synthetic), their thermal properties (thermoplastics vs thermosets), and the mechanisms of their formation (addition vs condensation polymerization).
10.4.1 Classification of Polymers
- Natural Polymers: Created by biological processes, including proteins (e.g., collagen), polysaccharides (e.g., cellulose), natural rubber, DNA, and RNA.
- Synthetic Polymers: Man-made through chemical processes, such as polyethylene, polystyrene, nylon, and Teflon.
10.4.2 Mechanisms of Polymerization
- Radical Polymerization: Initiation with radicals, followed by chain propagation and termination.
- Ionic Polymerization: Cationic or anionic processes lead to the formation and propagation of polymers.
- Coordination Polymerization: Utilizes transition metal catalysts for controlled polymer synthesis.
- Condensation Polymerization: Involves the reaction of monomers with two or more functional groups, releasing small molecules like water or HCl.
10.4.3 Polymer Structure and Properties
- Structures: Linear, branched, and crosslinked polymers exhibit varying properties.
- Crystallinity and Glass Transition: The arrangement of polymer chains influences density, melting points, and mechanical properties.
10.4.4 Major Classes of Synthetic Polymers and Applications
- Key synthetic polymers include:
- Polyethylene (PE): Used in packaging and containers.
- Polypropylene (PP): Used in automotive components and textiles.
- Polyvinyl Chloride (PVC): Durable and used in pipes and flooring.
- Polyesters (e.g., PET): Found in beverage bottles and textiles.
- Polyamides (Nylons): Utilized in textiles and engineering.
- Polymers with specialty applications, like biodegradable polymers (PLA, PHB) for sustainable use in products.
Each of these categories reveals the integral roles polymers fulfill and their significance in advancing technology and improving daily life.
Youtube Videos

Audio Book
Dive deep into the subject with an immersive audiobook experience.
Introduction to Polymers
Chapter 1 of 7
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Polymers are large macromolecules composed of repeating units called monomers. Organic polymers dominate many aspects of modern life: packaging, textiles, engineering plastics, rubber products, adhesives, coatings, and even biological molecules (proteins, nucleic acids).
Detailed Explanation
Polymers are large molecules made up of smaller, simple molecules called monomers that bond together in long chains. Examples of these chains can be found all around us, from the plastic wrapping that keeps our food fresh to the clothes we wear. Polymers are essential in many industries, such as packaging and textiles. Biological polymers include proteins and nucleic acids, which are vital for life.
Examples & Analogies
Think of polymers as long chains of beads, where each bead represents a monomer. Just as you can create a necklace by linking beads together in various ways, polymers are formed by linking monomers in a specific order. For example, when you use plastic wrap, you are using a polymer that is flexible and strong, just like a necklace made up of sturdy beads!
Classification of Polymers
Chapter 2 of 7
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
- Natural versus Synthetic.
Natural polymers are produced by living organisms and include: - Proteins: Polymers of amino acids linked by peptide bonds. Examples: collagen, keratin, enzymes.
- Polysaccharides: Polymers of monosaccharides linked by glycosidic bonds. Examples: cellulose, starch, glycogen.
- Natural rubber: Polyisoprene obtained from Hevea brasiliensis latex.
- DNA and RNA: Polymers of nucleotides.
Synthetic polymers are human-made through chemical polymerization processes. Examples include polyethylene, polypropylene, polyvinyl chloride, polystyrene, nylon, polyester, acrylics, and polytetrafluoroethylene (Teflon).
Detailed Explanation
Polymers can be broadly categorized into natural and synthetic types. Natural polymers occur in nature and are produced by living organisms, including proteins, polysaccharides, natural rubber, and DNA or RNA. Synthetic polymers, on the other hand, are created by chemical processes in laboratories or factories, leading to a variety of products such as plastics used in packaging, clothing, and even construction materials.
Examples & Analogies
You can think of natural polymers as the ingredients in a homemade soup, where each ingredient adds flavor and nutrition, while synthetic polymers are like prepared meals sold at a supermarket, ready to use but made in large-scale kitchens. Just as some people prefer cooking from scratch (natural) while others enjoy the convenience of pre-made meals (synthetic), industries use both natural and synthetic polymers based on their needs.
Thermoplastics vs. Thermosets
Chapter 3 of 7
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
- Thermoplastics versus Thermosets.
Thermoplastics soften upon heating and can be reshaped, then harden upon cooling. They are linear or lightly branched or lightly crosslinked polymers. Examples: polyethylene (PE), polypropylene (PP), polystyrene (PS), polyvinyl chloride (PVC), polyethylene terephthalate (PET).
Thermosets undergo irreversible curing (crosslinking) during processing; once set, they cannot be melted or reshaped. They are heavily crosslinked polymers. Examples: epoxies, phenolic resins, melamine formaldehyde, vulcanized rubber (crosslinked polyisoprene), polyester resins.
Detailed Explanation
Thermoplastics are types of polymers that become soft and pliable when heated, allowing them to be molded into different shapes. Once they cool down, they harden. Some common examples include plastics used in containers and bags. On the other hand, thermosets are polymers that undergo a chemical change when heated, forming a hard, inflexible material that cannot be remolded. They are more durable and heat-resistant, making them ideal for items like adhesives and coatings.
Examples & Analogies
Imagine thermoplastics like ice cream that melts when it gets warm – you can scoop it into different shapes or refill a cone, and once it refreezes, it hardens again. Thermosets, however, are like baked clay; once it’s set in the oven, you can’t reshape it. Just like you cannot turn baked clay back into soft clay, thermosets cannot revert to their original form after they have been cured.
Addition vs. Condensation Polymers
Chapter 4 of 7
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
- Addition (Chain-Growth) Polymers versus Condensation (Step-Growth) Polymers.
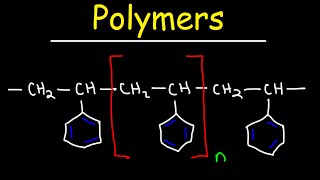
Addition (chain-growth) polymerization: Monomers with C=C (alkenes) or other unsaturated functional groups open up to form long chains without elimination of small molecules. Initiation (radical, cationic, or anionic) forms a reactive center, propagating by successive addition of monomers. Examples: polyethylene (from ethene), polypropylene (from propene), polyvinyl chloride (from vinyl chloride), polystyrene (from styrene).
Condensation (step-growth) polymerization: Monomers with two or more functional groups (e.g., –OH and –COOH) react to form covalent bonds, eliminating a small molecule such as H2O, HCl, or NH3. Polymer grows by successive steps between oligomeric units. Examples: Polyesters: e.g., polyethylene terephthalate (PET) from terephthalic acid and ethylene glycol; Polyamides: e.g., nylon 6,6 from hexamethylenediamine and adipic acid; Kevlar from p-phenylenediamine and terephthaloyl chloride (eliminating HCl).
Detailed Explanation
The process of making polymers can occur through addition (chain-growth) or condensation (step-growth) mechanisms. In addition polymerization, monomers join together with no loss of atoms, often involving double bonds being 'opened up'. This results in long chains of repeating units. For example, polyethylene is formed from the addition of ethylene molecules. In contrast, condensation polymerization involves the reaction of monomers with two functional groups. As these monomers bond, they release small molecules like water or hydrochloric acid. This process is common in the formation of complex polymers like nylon or polyester.
Examples & Analogies
Think of addition polymers as building a long chain using very long rubber bands (which stretch but do not break), while condensation polymers are like two people building a bridge by attaching large blocks together but losing small parts (like pebbles or sand) in the process. The rubber bands can easily be made longer or shorter, but the bridge is fixed once built, showing how addition can be flexible and reversible while condensation is more permanent.
Mechanisms of Polymerization
Chapter 5 of 7
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
10.4.2 Mechanisms of Polymerization. A. Radical Polymerization (Free-Radical Addition) 1. Initiation: Radical initiator (e.g., benzoyl peroxide, AIBN) decomposes thermally to form radicals. Radicals add to the monomer (alkene) to produce a new radical at the chain end. 2. Propagation: The chain-end radical reacts with additional monomer molecules, extending the chain one monomer unit at a time, generating a new radical at the end after each addition. 3. Termination: Two chain-end radicals combine (coupling) or disproportionate (a hydrogen atom transfers from one chain to another), quenching radical activity. 4. Chain transfer: The radical center transfers to another molecule (monomer, solvent, or polymer), which can lead to branched or lower-molecular-weight chains.
Detailed Explanation
Radical polymerization is a common method used to create polymers, and it proceeds through four main steps. Firstly, the initiation step activates a radical initiator, which starts the reaction by producing free radicals. These radicals react with monomers, allowing the chain to grow longer step-by-step. The process can conclude in various ways, such as two radicals combining together - a termination step. Another possible outcome is the radical transferring to another molecule, leading to different chain structures.
Examples & Analogies
You can think of this process like a game of tag. The person who is 'it' (the free radical) tags another player (the monomer), which then joins the group (grows the polymer). As more players are tagged, the group gets bigger. However, the game can 'end' when two tagged players decide to team up and stop the game (termination) or when one of the players passes their tag to someone else, creating a new group dynamic (chain transfer).
Polymer Structure and Properties
Chapter 6 of 7
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
10.4.3 Polymer Structure and Properties. 1. Linear versus branched versus crosslinked polymers.
- Linear polymers: Chains that are not interconnected; can pack closely, often crystalline or semicrystalline (e.g., HDPE). Tend to be strong and have high density.
- Branched polymers: Chains with side branches; cannot pack as tightly, often lower density and crystallinity (e.g., low-density polyethylene, LDPE). Branching arises from chain transfer during radical polymerization.
- Crosslinked polymers: Covalent bonds between polymer chains create a network. Low crosslink density yields elastomers (rubber-like elasticity); high crosslink density yields thermosets (rigid, heat-resistant materials).
Detailed Explanation
The structure of polymers affects their physical and chemical properties significantly. Linear polymers consist of long, unconnected chains that can be tightly packed, resulting in high strength and density. Branched polymers have side chains, leading to lower densities and less crystallinity. Crosslinked polymers feature covalent bonds linking different chains, creating networks. This network structure can give rise to rubbery materials or very hard, rigid products, depending on the extent of crosslinking.
Examples & Analogies
Imagine linear polymers as long ropes laid in a straight line, neatly packed together, creating a strong and resilient wall. Branched polymers would be like a messy pile of ropes tangled together, making it weaker and unable to support much weight. Crosslinked polymers are like a strong net made of ropes that are tied together; it can stretch and hold a lot of weight, but once it's set, it cannot be reshaped, similar to how thermosets work.
Major Classes of Synthetic Polymers and Applications
Chapter 7 of 7
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
10.4.4 Major Classes of Synthetic Polymers and Applications. 1. Polyethylene (PE)
- Monomer: Ethene (CH2=CH2).
- Variants:
- Low-density polyethylene (LDPE): Produced by free-radical polymerization under high pressure (1000–3000 atm) and high temperature (~200–300 °C). Highly branched, less crystalline, low density (0.91–0.93 g/cm³), flexible. Used in plastic bags, films, squeeze bottles.
- High-density polyethylene (HDPE): Produced by Ziegler–Natta or metallocene catalysts at lower pressure. Largely linear, highly crystalline, higher density (0.94–0.97 g/cm³), stronger and more rigid. Used in milk jugs, detergent bottles, piping, geomembranes.
Detailed Explanation
Polyethylene is one of the most widely used synthetic polymers, derived from the monomer ethylene. There are two main types of polyethylene - low-density polyethylene (LDPE) and high-density polyethylene (HDPE). LDPE is flexible and used in products like plastic bags due to its branching, while HDPE is stronger and stiffer due to its more linear structure, making it suitable for containers and piping. Each version serves different purposes based on its structural properties.
Examples & Analogies
Think of LDPE as a soft, bendable straw that can flex easily; it’s perfect for wrapping items without breaking. On the other hand, HDPE is like a sturdy broomstick, strong and rigid, making it ideal for items that need to hold more weight, like milk jugs or water pipes. Different structures lead to different uses, just like you wouldn’t use a broomstick to drink your juice!
Key Concepts
-
Polymers: Large macromolecules made from repeating monomer units.
-
Natural vs Synthetic Polymers: Natural polymers occur in nature, while synthetic ones are chemically created.
-
Thermoplastics vs Thermosets: Thermoplastics can be reshaped with heat, thermosets cannot.
-
Addition vs Condensation Polymerization: Addition polymerization does not result in byproducts, while condensation polymerization does.
Examples & Applications
Natural polymers include proteins like collagen and starches.
Synthetic polymers include polyethylene made from ethylene.
Thermoplastics such as polyvinyl chloride (PVC) can be reshaped when heated.
Thermosets like epoxy resin are typically used in adhesives and coatings.
Memory Aids
Interactive tools to help you remember key concepts
Rhymes
Polymers are cool, they bend and they break, some set like a stone, some melt for your sake.
Stories
Imagine a bakery where each ingredient represents a monomer. The mixing of flour, sugar, and eggs creates different baked goods, just like how monomers combine to make polymers.
Memory Tools
Remember 'PATCH' for polymer types: P for Plastics, A for Amino acids, T for Textiles, C for Chemical fibers, H for High-performance materials.
Acronyms
Think 'NATS' for Natural vs. Synthetic, Thermoplastic vs Thermoset.
Flash Cards
Glossary
- Polymer
A large molecule composed of repeating structural units called monomers.
- Monomer
The smallest unit of a polymer that can join with other monomers to form the polymer chain.
- Thermoplastic
A type of plastic that becomes moldable upon heating and can be reshaped multiple times.
- Thermoset
A polymer that irreversibly hardens upon heating and cannot be remolded.
- Addition Polymerization
A method of forming polymers by the addition of monomers with unsaturated bonds without the formation of byproducts.
- Condensation Polymerization
A process where two monomers react to form a polymer and a small molecule, such as water.
Reference links
Supplementary resources to enhance your learning experience.
