Elimination Reactions
Enroll to start learning
You’ve not yet enrolled in this course. Please enroll for free to listen to audio lessons, classroom podcasts and take practice test.
Interactive Audio Lesson
Listen to a student-teacher conversation explaining the topic in a relatable way.
Introduction to Elimination Reactions
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Welcome, everyone! Today we are diving into elimination reactions. Can anyone tell me what we mean by the term 'elimination' in organic chemistry?

Is it when something is removed from a molecule?

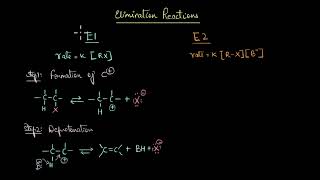
Exactly! Elimination reactions involve the removal of atoms or groups from adjacent carbon atoms, forming a new pi bond. This usually leads to the creation of unsaturated compounds, like alkenes or alkynes.

So, what's the difference between an elimination reaction and a substitution reaction?

Great question! In a substitution reaction, one atom or group replaces another, while in elimination, we’re removing groups to form double or triple bonds. Today, we’ll focus on two main types of elimination reactions: dehydration of alcohols and dehydrohalogenation of haloalkanes. We'll also touch on regioselectivity, like Zaitsev's Rule.
Dehydration of Alcohols
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Let’s start with the dehydration of alcohols. Can anyone explain what happens during this reaction?

Isn't it where water is removed to form an alkene?

Spot on! We typically use concentrated sulfuric acid or aluminum oxide as dehydrating agents and apply heat. The key result is the formation of an alkene. Can anyone tell me about the regioselectivity involved in this reaction?

I think it has something to do with Zaitsev's Rule, right?

Yes! Zaitsev's Rule states that the major product is the more substituted alkene. This means if there are multiple hydrogens we can remove, we prefer to take the one from the carbon with fewer hydrogens. For example, if we dehydrate butan-2-ol, what alkene would we expect as the major product?

That would be but-2-ene!
Dehydrohalogenation of Haloalkanes
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Moving on, let’s discuss dehydrohalogenation of haloalkanes. Can anyone tell me what this reaction entails?

It involves removing a hydrogen and a halogen from adjacent carbons to form an alkene, right?

Exactly! We need to use a strong base, like KOH or NaOH, but it must be in an alcoholic solution to prevent substitution reactions. Do you recall the necessary conditions for this reaction?

Heating under reflux?

Correct! The reaction proceeds better when heating is applied. Just like with dehydration, what type of regioselectivity applies here?

Zaitsev's Rule again!

Yes! So remember, Zaitsev's Rule helps us predict that the more substituted alkene will be the major product. For example, if we have bromoethane and react it with KOH, what alkene will we obtain?

That would be ethene!
Introduction & Overview
Read summaries of the section's main ideas at different levels of detail.
Quick Overview
Standard
This section covers elimination reactions, specifically dehydration of alcohols and dehydrohalogenation of haloalkanes. It emphasizes the formation of alkenes and the rules governing regioselectivity, such as Zaitsev's Rule, highlighting the conditions and products involved in these reactions.
Detailed
Elimination Reactions
Elimination reactions play a crucial role in organic chemistry by transforming saturated compounds into unsaturated compounds through the removal of atoms from adjacent carbon atoms. This process often results in the formation of a new pi (π) bond. Key points include:
- Dehydration of Alcohols: Involves the removal of a water molecule from an alcohol, leading to alkene formation. Concentrated sulfuric acid or aluminum oxide acts as a dehydrating agent, and heating is typically required.
- Regioselectivity: Zaitsev's Rule states that the more substituted alkene is the major product when unsymmetrical alcohols are dehydrated.
- Dehydrohalogenation of Haloalkanes: Involves the removal of a hydrogen atom and a halogen from adjacent carbons, leading to an alkene. A strong base in an alcoholic solvent is necessary for this reaction, combined with heating to drive it to completion.
- Regioselectivity: Similar to dehydration, Zaitsev's Rule applies, favoring the formation of the more substituted alkene as the major product.
Overall, elimination reactions are significant for the synthesis of alkenes and for understanding the mechanistic pathways in organic reactions.
Youtube Videos







Audio Book
Dive deep into the subject with an immersive audiobook experience.
Introduction to Elimination Reactions
Chapter 1 of 3
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Elimination reactions involve the removal of atoms or groups from adjacent carbon atoms within a molecule, resulting in the formation of a new pi (π) bond, typically leading to an unsaturated compound (an alkene or alkyne). A small molecule (like water or a hydrogen halide) is 'eliminated.'
Detailed Explanation
Elimination reactions are processes where parts of a molecule are removed, leading to the formation of a new multiple bond (like a double or triple bond). This often results in the transformation of a saturated compound (one without double bonds) into an unsaturated one (which has double or triple bonds). For example, when ethanol (an alcohol) loses a water molecule, it transforms into ethene, an alkene. This is a key type of reaction in organic chemistry because it allows the creation of alkenes and alkynes, which are important in various chemical syntheses.
Examples & Analogies
Think of it like taking a piece out of a puzzle. When you remove a piece (like water), the remaining pieces can fit together differently to form a new shape (like a double bond in ethene) that opens up new possibilities for attaching other pieces (further reactions).
Dehydration of Alcohols
Chapter 2 of 3
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Dehydration of Alcohols:
- Description: The removal of a molecule of water from an alcohol to form an alkene. This is the reverse of the hydration of alkenes.
- Reagents: Concentrated sulfuric acid (H2 SO4) or aluminum oxide (Al2 O3). These act as dehydrating agents.
- Conditions: Heating is required. The specific temperature can vary, e.g., 170 °C for H2 SO4.
- Products: Alkene and water.
- Regioselectivity: Zaitsev's Rule (Saytzeff's Rule): For unsymmetrical alcohols (where the removal of hydrogen can occur from more than one adjacent carbon), the major product is the alkene that has the greater number of alkyl groups attached to the double bond. This means the hydrogen is preferentially removed from the carbon atom with fewer hydrogen atoms (i.e., the most substituted alkene is the major product).
-
Example:
CH3CH2OH (ethanol) + Conc. H2SO4, 170 °C → CH2=CH2 (ethene) + H2O
Example:
CH3CH(OH)CH2CH3 (butan-2-ol) + Conc. H2SO4, heat → CH3CH=CHCH3 (but-2-ene, major product) + H2O
Detailed Explanation
During the dehydration of alcohols, a molecule of water is removed from the alcohol, resulting in the formation of an alkene. The regent, usually concentrated sulfuric acid, acts to facilitate this removal of water. The reaction is typically undertaken at elevated temperatures to ensure that the elimination occurs efficiently. According to Zaitsev's Rule, when there are multiple possible products, the reaction prefers to form the more substituted alkene, which is generally more stable due to greater alkyl group stabilization of the double bond.
Examples & Analogies
Imagine cooking a dish where you have to remove the steam (representing water) to concentrate the flavors. When making pasta, if you keep boiling it without a lid, excess water will evaporate, intensifying the flavor of the noodles (just like how dehydration intensifies the structural composition of a compound, creating a more complex molecule).
Dehydrohalogenation of Haloalkanes
Chapter 3 of 3
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Dehydrohalogenation of Haloalkanes:
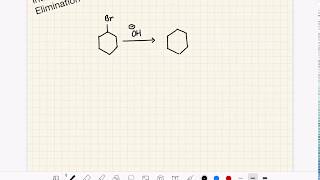
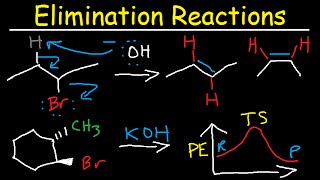
- Description: The removal of a hydrogen atom and a halogen atom from adjacent carbons of a haloalkane to form an alkene.
- Reagents: A strong base. Crucially, the base must be dissolved in an alcoholic (ethanolic) solvent (e.g., concentrated ethanolic KOH or NaOH). Using an aqueous solution of the base would primarily lead to nucleophilic substitution.
- Conditions: Heating under reflux is typically required to drive the reaction to completion.
- Products: Alkene, a salt, and water.
- Regioselectivity: Also follows Zaitsev's Rule for unsymmetrical haloalkanes, favouring the most substituted alkene.
-
Example:
CH3CH2Br (bromoethane) + KOH(alc), heat → CH2=CH2 (ethene) + KBr + H2O
Detailed Explanation
In the process of dehydrohalogenation, a hydrogen atom and a halogen atom (like bromine or chlorine) are removed from neighboring carbon atoms in a haloalkane. This results in the formation of a double bond, resulting in an alkene. This reaction requires a strong base and is typically performed in an alcoholic solution to ensure that elimination occurs rather than substitution. Zaitsev's Rule also dictates that the formation of the more substituted alkene will be favored during this reaction, producing a more stable molecule.
Examples & Analogies
Think of making a fruit salad by removing different fruits (just like removing atoms). If you’re making a salad, you might favor the more flavorful fruits (more substituted) to create a more delicious dish. In our reaction, we want to remove the less stable or less flavorful options (the halogen and hydrogen), leading to a more concentrated and appealing outcome (the alkene).
Key Concepts
-
Elimination Reactions: Essential reactions transforming saturated compounds into unsaturated ones.
-
Dehydration of Alcohols: Removal of water to form alkenes, using reagents like sulfuric acid.
-
Dehydrohalogenation: Involves removal of H and halogen to form alkenes in the presence of strong bases.
-
Zaitsev's Rule: Predominantly forms the more substituted alkene in elimination reactions.
-
Regioselectivity: Essential for predicting the outcome of elimination reactions.
Examples & Applications
Dehydration of ethanol (CH3CH2OH) with concentrated sulfuric acid gives ethene (CH2=CH2) and water.
Dehydrohalogenation of bromoethane (C2H5Br) with ethanolic KOH yields ethene and potassium bromide.
Memory Aids
Interactive tools to help you remember key concepts
Rhymes
In elimination we remove with haste, / To make alkenes, the substituents we waste.
Stories
Once upon a time, in a chemistry lab, a group of molecules was feeling cramped. They decided to stage a dramatic elimination, kicking out groups to create an open double bond and become more stable alkenes!
Memory Tools
For elimination reactions, remember: 'Remove Hydrogen and Halogen to gain the pi structuring!' (the last word signifies the formation of a pi bond).
Acronyms
Z-Rules - Zaitsev's Rule guides us to the most substituted alkene, Z for Zaitsev, R for Regioselectivity.
Flash Cards
Glossary
- Elimination Reaction
A reaction that involves the removal of atoms or groups from adjacent carbon atoms, resulting in the formation of a pi bond.
- Dehydration
The removal of a water molecule from an alcohol to form an alkene.
- Dehydrohalogenation
The removal of hydrogen and halogen from adjacent carbons of a haloalkane to form an alkene.
- Zaitsev's Rule
A rule stating that the major product of an elimination reaction is the more substituted alkene during dehydration of alcohols or dehydrohalogenation.
- Regioselectivity
The preference for the formation of one structural isomer over another in a reaction.
Reference links
Supplementary resources to enhance your learning experience.
