Substitution Reactions
Enroll to start learning
You’ve not yet enrolled in this course. Please enroll for free to listen to audio lessons, classroom podcasts and take practice test.
Interactive Audio Lesson
Listen to a student-teacher conversation explaining the topic in a relatable way.
Introduction to Substitution Reactions
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Welcome to today's class! Can anyone tell me what substitution reactions are?

Are they reactions where something gets replaced in a molecule?

Exactly! Substitution reactions involve replacing one atom or group in a compound with another. This is essential in organic chemistry for transforming molecules. Can anyone name an example?

Like when an alkane turns into a haloalkane?

Yes, that’s a great example! Alkanes can undergo substitution reactions with halogens under certain conditions. Can anyone summarize what those conditions are?

I think it requires high energy, like UV light, right?

Correct! Very insightful, everyone. Remember, understanding substitution reactions helps in predicting how organic compounds react and interact.
Free Radical Substitution in Alkanes
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Now, let’s talk about how alkanes undergo free radical substitution. Can anyone explain how this starts?

It starts with initiation, right? When halogen bonds break to form radicals?

Yes! That's the initiation step! What comes next in the substitution process?

The halogen radicals attack the alkane to form new radicals?

Exactly! That’s called propagation. And how does this process end?

It ends with termination when the radicals combine to form stable molecules.

Perfect! It’s crucial to remember these steps: initiation, propagation, and termination.
Nucleophilic Substitution in Haloalkanes
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Let’s shift gears. What about haloalkanes? How do substitution reactions occur here?

They undergo nucleophilic substitution where nucleophiles attack the carbon.

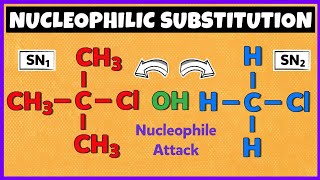
Great! Now, can anyone explain the difference between SN1 and SN2 mechanisms?

SN2 is a one-step reaction involving a backside attack, while SN1 is a two-step process that forms a carbocation.

Exactly right! In SN1, the formation of the carbocation is the rate-determining step, while in SN2, both reactants are involved in the slow step. Can anyone provide examples of nucleophiles used in these reactions?

Oh, hydroxide, cyanide, and ammonia can all act as nucleophiles!

Fantastic! Understanding these mechanisms will enhance your grasp of synthetic reactions in organic chemistry.
Introduction & Overview
Read summaries of the section's main ideas at different levels of detail.
Quick Overview
Standard
This section explores substitution reactions, particularly focusing on alkanes and haloalkanes. Substitution processes like free radical substitution in alkanes require high energy conditions, while nucleophilic substitution in haloalkanes involves the attack of nucleophiles on electrophilic carbon atoms, detailing the mechanisms, reagents, and products involved in these reactions.
Detailed
Substitution Reactions
Substitution reactions are crucial processes in organic chemistry that involve the exchange of one atom or group in a molecule with another. These reactions are predominantly observed in saturated compounds, such as alkanes and haloalkanes. In alkanes, substitution occurs via free radical mechanisms, typically activated under high-energy conditions such as ultraviolet light or high temperatures. This section elaborates on two primary types of substitution reactions: free radical substitution in alkanes and nucleophilic substitution in haloalkanes.
Substitution Reactions of Alkanes (Free Radical Substitution)
- Free radical substitution entails the replacement of hydrogen atoms with halogens in alkanes.
- The reaction begins with initiation, where halogen molecules dissociate into free radicals under UV light, followed by propagation steps where radicals react with alkanes, and concludes with termination steps that form stable products. This method often leads to a mixture of mono-, di-, and polysubstituted products due to the radical nature of the reaction.
Substitution Reactions of Haloalkanes (Nucleophilic Substitution)
- Haloalkanes serve as versatile substrates in nucleophilic substitution reactions, where nucleophiles attack the electrophilic carbon bonded to the halogen, leading to the displacement of the halogen group.
- The section distinguishes between SN1 and SN2 mechanisms based on the substitution reaction's pathways. The SN2 mechanism is characterized by a concerted process where bond-breaking and bond-forming occur simultaneously, while the SN1 mechanism involves a two-step process with the formation of a carbocation intermediate.
- Various nucleophiles such as hydroxide, cyanide, and ammonia are employed in these reactions with appropriate conditions to yield diverse organic compounds.
Youtube Videos

Audio Book
Dive deep into the subject with an immersive audiobook experience.
Overview of Substitution Reactions
Chapter 1 of 5
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Substitution reactions are characterized by the replacement of one atom or group in a molecule by another atom or group. These reactions are typical of saturated compounds (like alkanes and haloalkanes) and can also occur with functional groups that contain a good leaving group (such as the protonated hydroxyl group in alcohols).
Detailed Explanation
Substitution reactions involve swapping one part of a molecule for another. For example, in organic chemistry, this is common with alkanes (which are saturated hydrocarbons) and compounds that have good leaving groups like haloalkanes or alcohols. A leaving group is an atom or group that can easily depart with a pair of electrons, essentially allowing other groups to take their place. This type of reaction is crucial for creating a variety of organic compounds.
Examples & Analogies
Think of substitution reactions like a game of musical chairs. Just as players swap positions when the music stops, atoms or groups swap places in a molecule during a substitution reaction. For instance, in a game, one player leaves a chair (the leaving group) so that another player (the substitute) can take their place.
Substitution Reactions of Alkanes
Chapter 2 of 5
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
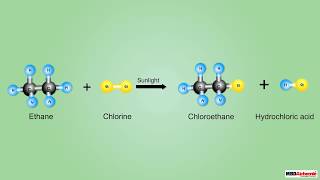
Substitution Reactions of Alkanes (Free Radical Substitution) Alkanes are generally quite unreactive due to their strong, nonpolar C-H and C-C sigma bonds. However, they can undergo substitution reactions with halogens under specific, high-energy conditions.
● Description: A hydrogen atom on the alkane is replaced by a halogen atom. Since this reaction involves highly reactive intermediates called free radicals, it is known as free radical substitution.
● Reagents: Halogens (e.g., Cl2, Br2).
● Conditions: Requires ultraviolet (UV) light (often sunlight or a UV lamp) or high temperatures (e.g., 300-400 °C). These conditions provide the energy needed to homolytically cleave the halogen bond to form free radicals.
● Products: Haloalkane and hydrogen halide. Due to the radical nature, it is often difficult to control the extent of substitution, leading to a mixture of mono-, di-, and polysubstituted products, as well as longer chain alkanes from radical coupling.
Detailed Explanation
Alkanes typically resist reactions because their bonds are very stable. However, they can react with halogens like chlorine or bromine under extreme conditions, such as exposure to UV light or high temperatures. This process is called free radical substitution. In this reaction, halogen atoms replace hydrogen atoms in the alkane. The reaction occurs in several stages: initiation (where free radicals are created), propagation (where free radicals react with alkanes), and termination (where two radicals combine to form a stable product).
Examples & Analogies
Imagine a light bulb as a metaphor for UV light in this reaction. A light bulb provides the energy necessary to spark a reaction, similar to how UV light energizes halogen molecules to break apart into radicals. When the radicals find an alkane, they can grab a hydrogen atom and leave a hole behind. This is like a pair of scissors cutting a piece of paper, where the scissors (radical) create an opening in the page (alkane), allowing something else (the halogen) to fit into that space.
Substitution Reactions of Haloalkanes
Chapter 3 of 5
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Substitution Reactions of Haloalkanes (Nucleophilic Substitution) Haloalkanes are extremely versatile starting materials in organic synthesis due to the polarity of the carbon-halogen bond. The electronegative halogen atom withdraws electron density from the carbon atom, making the carbon slightly positive (δ+) and thus susceptible to attack by nucleophiles. The halogen atom, when it leaves with its bonding electrons, is termed a leaving group.
● Description: A nucleophile (an electron-rich species, either a molecule with a lone pair or an anion) attacks the electrophilic carbon bonded to the halogen, displacing the halogen leaving group.
● Reagents: Various nucleophiles are used to synthesize different functional groups:
○ Hydroxide ion (OH−): To form alcohols.
○ Cyanide ion (CN−): To form nitriles.
○ Ammonia (NH3): To form amines.
○ Water (H2O): To form alcohols (slower reaction).
● Conditions: Typically involves heating under reflux in an aqueous or alcoholic solvent.
● Products: The halogen is replaced by the nucleophile.
Detailed Explanation
Haloalkanes, which are alkane molecules with halogen atoms attached, are reactive because the bond between carbon and halogen is polar. This means the carbon becomes slightly positive, making it a target for nucleophiles (electron-rich species). Nucleophiles attack the positively charged carbon, displacing the halogen (which acts as a leaving group) and forming a new compound. Different nucleophiles can lead to various products, like alcohols, amines, or nitriles, depending on the nucleophile used. This reaction often requires heating to drive the process to completion.
Examples & Analogies
Think of nucleophilic substitution like a game of tag in which the nucleophile is 'it.' The nucleophile (the tagger) can touch the haloalkane, which represents the carbon bonded to a halogen (the halogen acts as a player that leaves when tagged). Once the nucleophile tags the haloalkane and the halogen leaves, it changes the game, resulting in a new setup (a new compound). In this way, the reacting haloalkane transforms into another type of compound.
Mechanisms of Nucleophilic Substitution: SN1 and SN2
Chapter 4 of 5
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Mechanisms (HL): SN 1 and SN 2 The mechanism of nucleophilic substitution is highly dependent on the structure of the haloalkane (primary, secondary, or tertiary), the strength of the nucleophile, and the solvent.
○ SN 2 Mechanism (Substitution Nucleophilic Bimolecular)
○ SN 1 Mechanism (Substitution Nucleophilic Unimolecular)
Detailed Explanation
Nucleophilic substitution can happen through two primary mechanisms: SN1 and SN2. The SN2 mechanism involves a simultaneous bond-breaking and bond-forming process where the nucleophile attacks the carbon opposite the leaving group, leading to an inversion of configuration. This mechanism is favored by strong nucleophiles and occurs quickly, especially with primary haloalkanes, but slows down with tertiary ones due to steric hindrance. The SN1 mechanism is a two-step process that begins with the formation of a carbocation intermediate following the departure of the leaving group. This mechanism typically occurs with tertiary haloalkanes and is slower because it requires the formation of the carbocation. The rate of the reaction depends solely on the concentration of the haloalkane, rather than the nucleophile.
Examples & Analogies
Consider SN2 like a well-coordinated dance where each dancer must be perfectly timed to swap positions (bond formation and breaking occur together), while SN1 can be likened to a solo performance where the lead dancer (the carbocation) takes center stage before the backup dancers (nucleophiles) come in, resulting in a different dance configuration (the final product). The timing and arrangement of these dancers determine how smoothly the transition happens, similar to how the structure of the haloalkane affects the speed and outcome of the substitution.
Substitution Reactions of Alcohols
Chapter 5 of 5
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Substitution Reactions of Alcohols: The hydroxyl group (-OH) in alcohols is a very poor leaving group. To make it a good leaving group, it must first be converted into something more stable. This is typically achieved by protonation or reaction with specific halogenating agents.
● Description: The -OH group of an alcohol is replaced by a halogen atom to form a haloalkane.
● Reagents: Hydrogen Halides (HX): Concentrated HCl, HBr, or HI. Phosphorus Halides: PCl3, PCl5, PBr3. Thionyl Chloride (SOCl2).
● Conditions: Often requires heating.
● Products: Haloalkane and water (with HX), or other inorganic by-products.
Detailed Explanation
Alcohols feature a hydroxyl group that is not a great leaving group because it tends to leave as a strong base. To facilitate substitution, we need to convert the hydroxyl group into a form that can leave more easily, often by adding an acid to protonate it (turning it into -OH2+). Once it is a better leaving group, it can be replaced by a halogen using various reagents like hydrogen halides or phosphorus halides. The reaction typically involves heat to speed up the process, resulting in a haloalkane and additional products.
Examples & Analogies
Imagine the hydroxyl group in alcohols like a person trying to leave a party without their shoes on. It's uncomfortable (a poor leaving group) and makes it hard to go anywhere. If we help them put on a pair of slippers (protonation with acid), they can finally leave without difficulty (as a stable leaving group). This allows them to find a new spot (the haloalkane product) happily at a different party!
Key Concepts
-
Substitution Reactions: These involve the replacement of one atom or group in a molecule with another, crucial in organic transformations.
-
Free Radical Substitution: A type of substitution reaction that occurs in alkanes where free radicals attack and replace hydrogen atoms.
-
Nucleophilic Substitution: Involves the attack of nucleophiles on electrophilic carbon atoms in haloalkanes, leading to the displacement of halogens.
-
SN1 and SN2 Mechanisms: Different pathways for nucleophilic substitution characterized by their reaction rates and steps involved.
Examples & Applications
Example of Free Radical Substitution: The reaction of methane (CH4) with chlorine (Cl2) under UV light produces chloromethane (CH3Cl) and hydrogen chloride (HCl).
Example of Nucleophilic Substitution: The reaction of bromoethane (C2H5Br) with sodium hydroxide (NaOH) produces ethanol (C2H5OH) and bromide ion (Br-).
Memory Aids
Interactive tools to help you remember key concepts
Rhymes
Substitution, what a transformation, / One group goes, another takes its station!
Stories
The tale of the noble alkane, who invited halogens to replace its hydrogen friends under the bright UV light, turning into haloalkanes!
Memory Tools
RIP: R - Replace, I - Initiate, P - Propagate, to recall the steps in free radical substitution.
Acronyms
NARROW
- Nucleophile
- Attack
- Replaces
- Radical
- Organic
- Wades out of substitution. Helps remember nucleophilic substitution steps.
Flash Cards
Glossary
- Substitution Reaction
A reaction where one atom or group in a molecule is replaced by another atom or group.
- Free Radical Substitution
A substitution reaction involving radicals, typically occurring in alkanes under high-energy conditions.
- Nucleophilic Substitution
A substitution reaction where a nucleophile replaces a leaving group in a haloalkane.
- SN1 Mechanism
A two-step reaction mechanism that includes the formation of a carbocation intermediate.
- SN2 Mechanism
A one-step reaction mechanism where the nucleophile attacks the electrophile and forms a bond simultaneously while the leaving group departs.
- Nucleophile
An electron-rich species that can donate an electron pair to electrophiles.
- Leaving Group
An atom or group that departs from the parent molecule during a substitution reaction.
Reference links
Supplementary resources to enhance your learning experience.
