Oxidation and Reduction in Organic Chemistry
Enroll to start learning
You’ve not yet enrolled in this course. Please enroll for free to listen to audio lessons, classroom podcasts and take practice test.
Interactive Audio Lesson
Listen to a student-teacher conversation explaining the topic in a relatable way.
Understanding Oxidation and Reduction
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

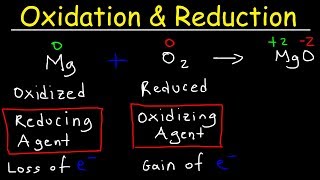
Let’s start with defining oxidation and reduction in organic chemistry. Oxidation can be described as gaining oxygen or losing hydrogen. Who can help me explain this further?

Does that mean when we oxidize a compound, it becomes more electronegative?

Exactly! And what about reduction?

Reduction would mean gaining hydrogen and losing oxygen, right?

Correct! A simple mnemonic to remember this is 'OIL RIG', meaning 'Oxidation Is Loss, Reduction Is Gain'.

So, if we think about it like that, every time we see a hydrogen being added, we can think it’s a reduction.

Yes, and vice versa with oxidation. Now, let’s summarize: Oxidation involves gaining oxygen or losing hydrogen; reduction involves gaining hydrogen or losing oxygen. Great job!
Functional Groups and Their Oxidation States
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Now let's observe how the oxidation states of various functional groups change. Starting from an alkane, can someone explain the oxidation path?

It goes from alkane to alcohol, then to aldehyde or ketone, and finally to carboxylic acid and CO2.

Exactly! This progression clearly shows each step in oxidation. What about their reductions?

We can reverse this order if we are reducing them. For instance, we can reduce a carboxylic acid back to an alcohol.

Fantastic! Knowing these pathways helps understand the reactivity of organic compounds. Remember, this is crucial for predicting reactions.
Common Oxidizing and Reducing Agents
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Let’s discuss the agents used in oxidation and reduction. What can you tell me about oxidizing agents?

Common oxidizing agents include potassium dichromate and potassium permanganate, right?

Correct! And what happens to potassium dichromate when it acts as an oxidizing agent?

It reduces from orange to green, showing that it has oxidized something.

That's a great observation! Now, can anyone tell me about the reducing agents we use?

Hydrogen gas is one, and so is lithium aluminium hydride, which is quite strong.

Exactly! Remember, understanding these agents and their behavior is key for mastering organic reactions.
Introduction & Overview
Read summaries of the section's main ideas at different levels of detail.
Quick Overview
Standard
In organic chemistry, oxidation refers to the gain of oxygen or loss of hydrogen, while reduction signifies the opposite. The section outlines common oxidizing and reducing agents, provides examples of oxidation states for different organic compounds, and emphasizes the sequences of oxidation and reduction reactions through practical chemical examples.
Detailed
In organic chemistry, the definitions of oxidation and reduction are broader than in inorganic contexts. Oxidation is characterized by the gain of oxygen, loss of hydrogen, or an increase in bonds to electronegative elements, while reduction entails losing oxygen, gaining hydrogen, or decreasing such bonds. The section establishes a clear relationship among various functional groups, showing how their oxidation states can be transformed sequentially, for instance, through oxidation of alkanes to carboxylic acids or carbon dioxide. Moreover, it identifies common agents such as acidified potassium dichromate and permanganate for oxidation, and hydrogen gas or lithium aluminium hydride for reduction. This framework is critical for understanding reactivity and transformations that organic compounds undergo in both laboratory and industrial settings.
Youtube Videos



Audio Book
Dive deep into the subject with an immersive audiobook experience.
Definitions in Organic Chemistry
Chapter 1 of 5
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Definitions in Organic Chemistry:
Oxidation:
- Gain of oxygen atoms.
- Loss of hydrogen atoms.
- Increase in the number of bonds to oxygen or other electronegative atoms (e.g., nitrogen, halogens).
Reduction:
- Loss of oxygen atoms.
- Gain of hydrogen atoms.
- Decrease in the number of bonds to oxygen or other electronegative atoms.
Detailed Explanation
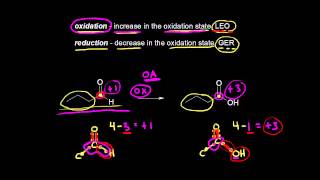
In organic chemistry, oxidation and reduction are defined in terms that differ slightly from those used in inorganic chemistry. Oxidation refers to processes where a molecule gains oxygen, loses hydrogen, or forms more bonds with electronegative atoms. Conversely, reduction involves losing oxygen, gaining hydrogen, or forming fewer bonds with electronegative elements.
For example, when converting alkanes to alcohols, the addition of oxygen indicates oxidation, while the removal of oxygen marks reduction.
Examples & Analogies
Think of oxidation and reduction like a balance concerning oxygen and hydrogen. Suppose you have a balloon that expands (gains oxygen) as you blow air into it—this is like oxidation. On the other hand, if you let air out (indicating a loss of oxygen), it shrinks—this is like reduction. Each process represents a change in state concerning those elements.
Examples of Oxidation/Reduction 'Levels'
Chapter 2 of 5
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Examples of Oxidation/Reduction 'Levels':
More reduced ↔ More oxidized
- Alkane → Alcohol → Aldehyde/Ketone → Carboxylic Acid → Carbon Dioxide (CO2) (Each step to the right is an oxidation; each step to the left is a reduction)
Detailed Explanation
The transformation from an alkane to carbon dioxide shows a series of oxidation states. At the far left, you have the alkane, which is less oxidized (more reduced). As it undergoes oxidation through each stage (alcohol, aldehyde, etc.), the number of bonds to more electronegative atoms increases, indicating a higher oxidation state. This series exemplifies how molecules can gain oxygen and lose hydrogen, illustrating the transitions from a reduced to an oxidized state.
Examples & Analogies
Consider this series like climbing a mountain. Starting at the base (alkane), you ascend (oxidize) through various plant types (alcohols and aldehydes) until you reach the peak (carbon dioxide). As you climb higher, you gather more gear to help you adapt—this is similar to gaining oxidation states as bonds with oxygen increase.
Common Oxidizing Agents
Chapter 3 of 5
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Common Oxidizing Agents:
- Acidified Potassium Dichromate(VI) (K2 Cr2 O7 /H+): An orange solution that turns green as Cr2 O72− is reduced to Cr3+. It's a versatile oxidizing agent, used for mild to strong oxidation depending on conditions (distillation vs. reflux).
- Acidified Potassium Permanganate(VII) (KMnO4 /H+): A strong purple oxidizing agent that turns colourless as MnO4− is reduced to Mn2+. Can be very vigorous.
- Alkaline Potassium Permanganate(VII) (KMnO4 /OH−): A purple solution that turns into a brown precipitate (MnO2) upon reduction. Used for milder oxidations.
- Tollen's Reagent: An ammoniacal silver nitrate solution that oxidizes aldehydes to form a silver mirror.
- Fehling's Solution / Benedict's Solution: Solutions containing copper(II) ions in an alkaline medium that oxidize aldehydes.
Detailed Explanation
These common oxidizing agents are pivotal in organic chemistry for facilitating various oxidation reactions. For instance, potassium dichromate changes color from orange to green when it oxidizes organic compounds. Tollen's reagent is particularly useful in distinguishing aldehydes since they can reduce silver ions, resulting in a noticeable silver mirror effect in laboratory settings.
Examples & Analogies
Imagine oxidizing agents as tools in a toolbox for a craftsman. Each tool (oxidizing agent) has its specific purpose and strength. Just as a hammer can drive a nail but can also pull it out (Tollen's reagent helping in specific reactions), different oxidizing agents can selectively oxidize molecules depending on their specific conditions and the substrate's nature.
Common Reducing Agents
Chapter 4 of 5
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Common Reducing Agents:
- Hydrogen gas (H2) with a catalyst (Ni, Pt, Pd): Used for hydrogenation of alkenes and alkynes to alkanes.
- Lithium Aluminium Hydride (LiAlH4): A powerful reducing agent that reduces aldehydes, ketones, carboxylic acids to alcohols.
- Sodium Borohydride (NaBH4): A milder and more selective reducing agent that reduces aldehydes and ketones to alcohols.
Detailed Explanation
Reducing agents are substances that donate electrons or hydrogen to other compounds, effectively reducing their oxidation state. For instance, lithium aluminium hydride is a strong reducing agent that can transform esters and carboxylic acids into alcohols, while sodium borohydride is more selective and typically can only reduce aldehydes and ketones.
Examples & Analogies
Think of reducing agents like a friend who's excellent at helping others lift heavy boxes. Hydrogen gas helps oil 'lift' and turn into fat, much like how these agents 'lift' electron counts to help reduce the compounds. Sodium borohydride, being the cautious friend, knows when to help and when to step back to avoid overdoing it!
Summary of Oxidation/Reduction Reactions by Functional Group
Chapter 5 of 5
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Summary of Oxidation/Reduction Reactions by Functional Group:
- Alkanes: Undergo complete combustion to form CO2 and H2O.
- Alkenes/Alkynes:
- Oxidation: Converts to diols or undergoes oxidative cleavage.
- Reduction: Converted to alkanes via hydrogenation.
- Alcohols:
- Primary (1°) Alcohols: Can oxidize to aldehydes or carboxylic acids.
- Secondary (2°) Alcohols: Oxidized to ketones.
- Tertiary (3°) Alcohols: Resistant to oxidation under normal conditions.
- Aldehydes: Easy oxidation to carboxylic acids.
- Ketones: Generally resistant to oxidation, can be reduced to secondary alcohols.
- Carboxylic Acids: Can be reduced to primary alcohols.
- Esters: Reduced to two alcohols by strong reducing agents.
Detailed Explanation
This summary provides a concise overview of how each functional group behaves during oxidation and reduction processes. Alkanes, for instance, undergo oxidation when combusted, while alcohols can transition into aldehydes or acids based on the agent used. Understanding these transformations is vital for predicting the products of organic reactions and using them wisely in synthetic chemistry.
Examples & Analogies
Imagine a shop where different types of fruits are processed. Alkanes (like apples) are transformed to a final product (like juice, CO2, and H2O) when completely oxidized. Alcohols (like pears) can turn into various products depending on the current 'season' (which tells us what oxidizing agent is in use). Each fruit represents different pathways and reactions in organic chemistry, making it essential to understand the behavior of each one.
Key Concepts
-
Oxidation: Gain of oxygen or loss of hydrogen; increases the number of bonds to electronegative atoms.
-
Reduction: Loss of oxygen or gain of hydrogen; decreases the number of bonds to electronegative atoms.
-
Common oxidizing agents include potassium dichromate and permanganate.
-
Common reducing agents include hydrogen gas and lithium aluminium hydride.
Examples & Applications
More reduced ↔ More oxidized
Alkane → Alcohol → Aldehyde/Ketone → Carboxylic Acid → Carbon Dioxide (CO2) (Each step to the right is an oxidation; each step to the left is a reduction)
Detailed Explanation: The transformation from an alkane to carbon dioxide shows a series of oxidation states. At the far left, you have the alkane, which is less oxidized (more reduced). As it undergoes oxidation through each stage (alcohol, aldehyde, etc.), the number of bonds to more electronegative atoms increases, indicating a higher oxidation state. This series exemplifies how molecules can gain oxygen and lose hydrogen, illustrating the transitions from a reduced to an oxidized state.
Real-Life Example or Analogy: Consider this series like climbing a mountain. Starting at the base (alkane), you ascend (oxidize) through various plant types (alcohols and aldehydes) until you reach the peak (carbon dioxide). As you climb higher, you gather more gear to help you adapt—this is similar to gaining oxidation states as bonds with oxygen increase.
--
Chunk Title: Common Oxidizing Agents
Chunk Text: ### Common Oxidizing Agents:
Acidified Potassium Dichromate(VI) (K2 Cr2 O7 /H+): An orange solution that turns green as Cr2 O72− is reduced to Cr3+. It's a versatile oxidizing agent, used for mild to strong oxidation depending on conditions (distillation vs. reflux).
Acidified Potassium Permanganate(VII) (KMnO4 /H+): A strong purple oxidizing agent that turns colourless as MnO4− is reduced to Mn2+. Can be very vigorous.
Alkaline Potassium Permanganate(VII) (KMnO4 /OH−): A purple solution that turns into a brown precipitate (MnO2) upon reduction. Used for milder oxidations.
Tollen's Reagent: An ammoniacal silver nitrate solution that oxidizes aldehydes to form a silver mirror.
Fehling's Solution / Benedict's Solution: Solutions containing copper(II) ions in an alkaline medium that oxidize aldehydes.
Detailed Explanation: These common oxidizing agents are pivotal in organic chemistry for facilitating various oxidation reactions. For instance, potassium dichromate changes color from orange to green when it oxidizes organic compounds. Tollen's reagent is particularly useful in distinguishing aldehydes since they can reduce silver ions, resulting in a noticeable silver mirror effect in laboratory settings.
Real-Life Example or Analogy: Imagine oxidizing agents as tools in a toolbox for a craftsman. Each tool (oxidizing agent) has its specific purpose and strength. Just as a hammer can drive a nail but can also pull it out (Tollen's reagent helping in specific reactions), different oxidizing agents can selectively oxidize molecules depending on their specific conditions and the substrate's nature.
--
Chunk Title: Common Reducing Agents
Chunk Text: ### Common Reducing Agents:
Hydrogen gas (H2) with a catalyst (Ni, Pt, Pd): Used for hydrogenation of alkenes and alkynes to alkanes.
Lithium Aluminium Hydride (LiAlH4): A powerful reducing agent that reduces aldehydes, ketones, carboxylic acids to alcohols.
Sodium Borohydride (NaBH4): A milder and more selective reducing agent that reduces aldehydes and ketones to alcohols.
Detailed Explanation: Reducing agents are substances that donate electrons or hydrogen to other compounds, effectively reducing their oxidation state. For instance, lithium aluminium hydride is a strong reducing agent that can transform esters and carboxylic acids into alcohols, while sodium borohydride is more selective and typically can only reduce aldehydes and ketones.
Real-Life Example or Analogy: Think of reducing agents like a friend who's excellent at helping others lift heavy boxes. Hydrogen gas helps oil 'lift' and turn into fat, much like how these agents 'lift' electron counts to help reduce the compounds. Sodium borohydride, being the cautious friend, knows when to help and when to step back to avoid overdoing it!
--
Chunk Title: Summary of Oxidation/Reduction Reactions by Functional Group
Chunk Text: ### Summary of Oxidation/Reduction Reactions by Functional Group:
Alkanes: Undergo complete combustion to form CO2 and H2O.
Alkenes/Alkynes:
Oxidation: Converts to diols or undergoes oxidative cleavage.
Reduction: Converted to alkanes via hydrogenation.
Alcohols:
Primary (1°) Alcohols: Can oxidize to aldehydes or carboxylic acids.
Secondary (2°) Alcohols: Oxidized to ketones.
Tertiary (3°) Alcohols: Resistant to oxidation under normal conditions.
Aldehydes: Easy oxidation to carboxylic acids.
Ketones: Generally resistant to oxidation, can be reduced to secondary alcohols.
Carboxylic Acids: Can be reduced to primary alcohols.
Esters: Reduced to two alcohols by strong reducing agents.
Detailed Explanation: This summary provides a concise overview of how each functional group behaves during oxidation and reduction processes. Alkanes, for instance, undergo oxidation when combusted, while alcohols can transition into aldehydes or acids based on the agent used. Understanding these transformations is vital for predicting the products of organic reactions and using them wisely in synthetic chemistry.
Real-Life Example or Analogy: Imagine a shop where different types of fruits are processed. Alkanes (like apples) are transformed to a final product (like juice, CO2, and H2O) when completely oxidized. Alcohols (like pears) can turn into various products depending on the current 'season' (which tells us what oxidizing agent is in use). Each fruit represents different pathways and reactions in organic chemistry, making it essential to understand the behavior of each one.
--
Memory Aids
Interactive tools to help you remember key concepts
Rhymes
Oxidation is gaining O, reduction's losing it, that’s how we flow!
Stories
Imagine a hero named Oxidation who always wants to gain oxygen, while his friend Reduction tries to lose it. They go on adventures through chemistry changes, showing us how compounds transform!
Memory Tools
Remember 'OIL RIG' for Oxidation Is Loss, Reduction Is Gain.
Acronyms
Use 'O and R' to stand for Oxidation and Reduction in reactions.
Flash Cards
Glossary
- Oxidation
A chemical reaction involving the gain of oxygen or loss of hydrogen, increasing bonds to electronegative atoms.
- Reduction
A chemical reaction involving loss of oxygen or gain of hydrogen, decreasing bonds to electronegative atoms.
- Oxidizing Agent
A substance that causes oxidation by accepting electrons or providing oxygen.
- Reducing Agent
A substance that causes reduction by donating electrons or providing hydrogen.
Reference links
Supplementary resources to enhance your learning experience.
