Calculations Involving Equilibrium Constants
Enroll to start learning
You’ve not yet enrolled in this course. Please enroll for free to listen to audio lessons, classroom podcasts and take practice test.
Interactive Audio Lesson
Listen to a student-teacher conversation explaining the topic in a relatable way.
Calculating K from Equilibrium Concentrations
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Today, we're going to learn about calculating the equilibrium constant, K, from equilibrium concentrations. Can anyone tell me what the equilibrium expression for a general reversible reaction looks like?

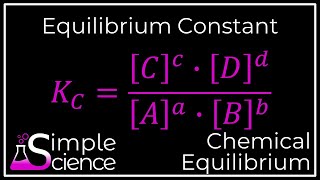
Isn't it Kc equals the concentration of products over the concentration of reactants raised to the power of their coefficients?

Exactly! For the reaction aA + bB ⇌ cC + dD, we can write Kc = [C]^c [D]^d / ([A]^a [B]^b). Now, let's do an example. If we have 0.20 M N₂, 0.30 M H₂, and 0.10 M NH₃, how would we find Kc for N₂ + 3H₂ ⇌ 2NH₃?

We substitute those concentrations into the expression, right?

Absolutely! And remember to square the concentration of NH₃ since it has a coefficient of 2. Can anyone perform the calculation now?

So Kc would be (0.10^2) / (0.20 * (0.30^3)) which equals approximately 1.85.

Great job! That's how we find K from experimentally measured concentrations.
Using ICE Tables for Equilibrium Calculations
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Now let’s move on to how to calculate equilibrium concentrations using ICE tables. Can anyone explain what ICE stands for?

ICE stands for Initial, Change, Equilibrium!

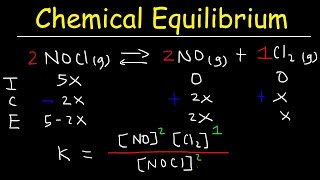
Correct! It helps us organize our data. For instance, let's take the decomposition of PCl₅ into PCl₃ and Cl₂ with Kc = 0.024 and initial concentration of PCl₅ as 0.100 M. What do we do first?

We set up our ICE table! The initial concentration of PCl₅ is 0.100 M, and PCl₃ and Cl₂ start at 0 M.

Exactly! And how would the concentrations change as they reach equilibrium if we let 'x' be the change in concentration for PCl₅?

The change for PCl₅ would be -x, and PCl₃ and Cl₂ would each gain x.

Good! Now write the equilibrium expression and plug in the values. What’s next?

We can solve for x using the Kc equation, and if needed, apply the quadratic formula.

Right! Remember to check if the approximation method is valid. Excellent work, team!
Approximation Method
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Finally, let's discuss the approximation method. When can we use this technique?

We can use it when K is very small, making x negligible compared to the initial concentrations!

Precisely! For example, if K is 0.001 in a system with initial concentration of 0.100, we can approximate 0.100 - x as just 0.100. Who can summarize the validity check for this approximation?

We need to ensure that x is less than 5% of the initial concentration!

Excellent! Understanding when to apply this method saves us a lot of complex calculations. Remember, always validate your assumptions!
Introduction & Overview
Read summaries of the section's main ideas at different levels of detail.
Quick Overview
Standard
In this section, we explore two main scenarios for calculations involving equilibrium constants: determining K from experimental equilibrium concentrations and predicting equilibrium concentrations using initial concentrations and K values through ICE tables. Real-world examples guide the understanding of these concepts.
Detailed
Detailed Summary of Section 6.3: Calculations Involving Equilibrium Constants
This section elaborates on the calculations involving equilibrium constants (K), detailing two main scenarios:
1. Calculating K from Equilibrium Concentrations/Partial Pressures: This straightforward process involves substituting experimentally determined equilibrium concentrations or partial pressures of reactants and products into the equilibrium expression to find the value of K.
Example: For the equilibrium reaction N₂(g) + 3H₂(g) ⇌ 2NH₃(g), if we have equilibrium concentrations of the components, we can directly plug in these values to compute Kc.
- Calculating Equilibrium Concentrations/Partial Pressures from Initial Conditions and K (using ICE Tables): This calculation involves establishing an ICE (Initial, Change, Equilibrium) table when initial concentrations and K are known. The changes in concentrations are denoted by a variable (often 'x'), allowing for the calculation of equilibrium concentrations as the system approaches equilibrium.
Example: In the case of PCl₅ decomposing into PCl₃ + Cl₂, when given initial conditions and Kc, the ICE table helps organize initial amounts, changes in concentration, and final equilibrium values. If K is small, an approximation method can simplify the calculations to avoid complex equations.
This section is significant as it builds foundational skills for solving equilibrium problems in chemistry, vital for understanding dynamic systems and reaction behaviors.
Youtube Videos








Audio Book
Dive deep into the subject with an immersive audiobook experience.
Calculating K from Equilibrium Concentrations
Chapter 1 of 3
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Calculations involving equilibrium constants typically involve two main scenarios: determining the value of K from experimental equilibrium concentrations, or predicting equilibrium concentrations given an initial setup and the value of K.
Scenario 1: Calculating the Value of K from Equilibrium Concentrations/Partial Pressures
This is the most straightforward calculation. If the equilibrium concentrations or partial pressures of all reactants and products are experimentally determined, these values can be directly substituted into the equilibrium constant expression.
Example 1: Calculating Kc
A 2.0 dm³ flask contains 0.40 mol of N₂, 0.60 mol of H₂, and 0.20 mol of NH₃ at equilibrium at a certain temperature. Calculate Kc for the reaction:
N₂(g) + 3H₂(g) ⇌ 2NH₃(g)
- Write the Kc expression:
Kc = [NH₃]² / ([N₂] [H₂]³) - Calculate equilibrium concentrations:
[N₂] = 0.40 mol / 2.0 dm³ = 0.20 mol dm⁻³
[H₂] = 0.60 mol / 2.0 dm³ = 0.30 mol dm⁻³
[NH₃] = 0.20 mol / 2.0 dm³ = 0.10 mol dm⁻³ - Substitute values into the expression:
Kc = (0.10)² / ((0.20) × (0.30)³)
Kc = 0.0100 / (0.20 × 0.027)
Kc = 0.0100 / 0.0054 = 1.85 (to 3 significant figures)
Detailed Explanation
In this scenario, we are learning how to calculate the equilibrium constant, K, using the concentrations or pressures of reactants and products that have been experimentally determined. The example provided demonstrates the steps:
1. Kc Expression: We start by writing the Kc expression for the reaction, which relates the concentrations of products to reactants raised to their stoichiometric coefficients.
2. Equilibrium Concentrations: Next, we determine the concentrations of reactants and products at equilibrium by dividing the amount (in moles) by the volume of the system (in dm³).
3. Substitution and Calculation: Finally, we substitute these values into the Kc expression and perform the calculations to find the value of Kc, which helps understand the extent of the reaction at equilibrium.
Examples & Analogies
Imagine you are mixing ingredients in a recipe. The amounts of each ingredient correspond to the concentrations of reactants. By determining how much of each ingredient is present after the cooking process (equilibrium), you can gauge how successful your recipe was (K value). If your dish tastes great (K is high), it means that the reactions of the ingredients produced a lot of flavorful components. On the other hand, if your dish doesn't taste very good (K is low), it suggests that not much of the flavorful compounds were made.
Calculating Equilibrium Concentrations with ICE Tables
Chapter 2 of 3
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Scenario 2: Calculating Equilibrium Concentrations/Partial Pressures from Initial Conditions and K (ICE Tables)
When you are given initial concentrations and the value of K, you need to determine how the concentrations will change to reach equilibrium. This is commonly done using an ICE (Initial, Change, Equilibrium) table.
Example 2: Calculating Equilibrium Concentrations using an ICE Table (Simple Case)
Consider the decomposition of PCl₅: PCl₅(g) ⇌ PCl₃(g) + Cl₂(g). At a certain temperature, Kc = 0.024. If 1.00 mol of PCl₅ is placed in a 10.0 dm³ container, calculate the equilibrium concentrations.
- Initial Concentrations:
[PCl₅] = 1.00 mol / 10.0 dm³ = 0.100 mol dm⁻³
[PCl₃] = 0 mol dm⁻³
[Cl₂] = 0 mol dm⁻³ - Set up the ICE Table: Let 'x' be the change in concentration of PCl₅.
Concentration (mol dm⁻³)
PCl₅ PCl₃ Cl₂
Initial (I) 0.100 0 0
Change (C) -x +x +x
Equilibrium (E) 0.100-x x x - Write the Kc expression:
Kc = [PCl₃][Cl₂] / [PCl₅] - Substitute equilibrium expressions into Kc and solve for x:
0.024 = (x)(x) / (0.100 - x)
0.024 (0.100 - x) = x² - Solve the quadratic equation:
x² + 0.024x - 0.0024 = 0
Using the quadratic formula, calculate x and then determine equilibrium concentrations.
Detailed Explanation
In this part, we address how to find equilibrium concentrations when given initial conditions and the value of K. The process involves the ICE method:
1. Initial Concentrations: We start by calculating the initial concentrations of the substances involved in the reaction based on the provided information.
2. Setting Up the ICE Table: An ICE Table is created where 'I' stands for initial concentrations, 'C' for the changes that occur, and 'E' for the equilibrium concentrations, allowing us to visualize the changes as the reaction progresses.
3. Kc Expression: The Kc expression is written, linking the concentrations at equilibrium.
4. Substituting Values: We substitute the equilibrium expressions derived from the ICE Table into the Kc expression to formulate an equation, often leading to a quadratic equation.
5. Solving the Quadratic: Finally, we use the quadratic formula to find the value of 'x', which tells us how much of each substance changes, allowing us to calculate the final equilibrium concentrations.
Examples & Analogies
Think of ICE Tables like tracking the inventory of a store: you start by noting how many of each item you have (Initial). As items are sold (Change), you record those changes. Finally, after a period of selling, you check how many items remain (Equilibrium). This systematic approach helps store managers (like chemists) understand how resources shift over time during sales (or chemical reactions).
Real-Life Applications and Approximations
Chapter 3 of 3
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Approximation Method:
If the value of K is very small (typically K < 10⁻³ or 10⁻⁴) and the initial concentration of reactants is relatively large, you can often make the approximation that 'x' is negligible compared to the initial concentration. For example, in 0.100 - x, if x is very small, 0.100 - x ≈ 0.100. This simplifies the calculation by avoiding the quadratic formula. After solving for x with the approximation, you must check if x is less than 5% of the initial concentration. If it is, the approximation is valid. If not, the quadratic formula must be used. In the example above, x (0.0384) is not negligible compared to 0.100 (it's 38.4%), so the approximation would not be valid.
Detailed Explanation
This section focuses on the approximation method often utilized in equilibrium calculations, especially when dealing with very small K values. The key points are:
1. When to Approximate: If K is small, indicating the reaction does not favor products, we can simplify our calculations. This is especially useful when concentrations of reactants are high relative to the changes expected.
2. The Approximation: We substitute the initial concentration into calculations instead of adjusting for 'x' (the change), making it easier to solve.
3. Validity Check: It's essential to validate the approximation by checking if 'x' is less than 5% of the initial concentration. If it is, our simplified calculation is reasonable; if not, we need to revert to using the full quadratic formula for accuracy.
Examples & Analogies
Consider planning a party: if you start with 100 guests and expect only a few (say, 5) to decline, you don't need to adjust your entire planning for those that might not show up. You can consider 95 guests as still attending (valid approximation). But if you expect half of them to decline (say, 50), that significantly changes everything, and you'd need to reconsider your arrangements. Similarly, in chemistry, small changes (like x) compared to large quantities allow simplifications in calculations, but larger changes require more precise methods.
Key Concepts
-
Equilibrium Constant (K): A measure of the ratio of products to reactants at equilibrium.
-
ICE Table: A valuable tool for calculating equilibrium concentrations by organizing initial, change, and equilibrium amounts.
-
Kc and its Calculation: The method for calculating Kc using equilibrium concentrations of reactants and products.
-
Approximation Method: A simplification technique applicable when K is small compared to initial concentrations.
Examples & Applications
Example of calculating Kc from given concentrations: For the reaction N₂ + 3H₂ ⇌ 2NH₃, if you know concentrations of N₂ as 0.2 M, H₂ as 0.3 M, and NH₃ as 0.1 M, you can compute Kc as 1.85.
Example of using an ICE table: For PCl₅ ⇌ PCl₃ + Cl₂, with K = 0.024 and initial PCl₅ = 0.1 M, we set up an ICE table to find equilibrium concentrations.
Memory Aids
Interactive tools to help you remember key concepts
Rhymes
In ICE tables, we see, Initial, Change, and Equilibrium key, makes finding K simpler, you’ll agree!
Stories
Once upon a time, in a land of chemicals, there lived an ICE table. It loved to help chemists remember their Initial amounts, watch the Change occur, and see them settle into a peaceful Equilibrium.
Memory Tools
Remember K: 'Keep Products Possible' – High K values indicate a push towards products at equilibrium!
Acronyms
K = 'King of rationing Products and Reactants'; when K is greater than one, products rule the equilibrium kingdom!
Flash Cards
Glossary
- Equilibrium Constant (K)
A numerical value that describes the ratio of products to reactants at equilibrium.
- ICE Table
A table used to organize initial concentrations, changes in concentrations, and equilibrium concentrations.
- Kc
Equilibrium constant expressed in terms of molar concentrations of reactants and products.
- Quadratic Formula
A formula used to find the roots of a quadratic equation, expressed as x = [-b ± sqrt(b² - 4ac)] / 2a.
- Approximation Method
A technique used to simplify equilibrium calculations when K is very small relative to initial concentrations.
Reference links
Supplementary resources to enhance your learning experience.
