Le Chatelier's Principle
Enroll to start learning
You’ve not yet enrolled in this course. Please enroll for free to listen to audio lessons, classroom podcasts and take practice test.
Interactive Audio Lesson
Listen to a student-teacher conversation explaining the topic in a relatable way.
Dynamic Equilibrium
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Today we are discussing dynamic equilibrium. What do you all think it means?

I think it means the reaction is stopped.

Not quite! Dynamic equilibrium means that even though it looks like the reaction has stopped, the forward and reverse reactions are still happening at equal rates.

So it’s like a balance?

Exactly! Just remember the phrase 'dynamic balance' to help you think of it that way. Can anyone name the characteristics of dynamic equilibrium?

I remember something about it being constant!

Great! At equilibrium, observable properties remain constant over time. Let’s summarize the key points: dynamic nature, constant macroscopic properties, achieved in a closed system, and reversible responses are all important.
Effects of Changes on Equilibrium
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Let’s delve into how changes affect equilibrium. What happens when we add more reactant?

The reaction should move towards the products, right?

Correct! This pertains to Le Chatelier's principle. If you remember, you can use the acronym ACE: Add = Concentrate on products, which helps recall this idea.

And if we remove a product?

Good question! According to Le Chatelier's Principle, if we remove a product, the reaction will shift to the left to replenish it.

What about pressure changes?

Pressure changes are significant for gaseous reactions. Decreasing the volume increases pressure, which favors the side with fewer moles of gas.

Can we summarize how it shifts with pressure?

Absolutely! For pressure, the key points are: increase pressure = shift to fewer moles, decrease pressure = shift to more moles.
Impact of Temperature and Catalysts
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Now let’s discuss temperature. What happens if we increase it?

Does the equilibrium shift toward the endothermic direction?

Yes! Remember the phrase 'Heat Equals Endothermic.' This is crucial in understanding temperature shifts. Can someone explain what happens if we add a catalyst?

It speeds up the reaction but doesn’t change equilibrium, right?

Exactly! Catalysts maintain the same equilibrium position but help the system reach it faster. Let’s recap temperature effects: increase temperature favors endothermic reactions and decrease favors exothermic.
Introduction & Overview
Read summaries of the section's main ideas at different levels of detail.
Quick Overview
Standard
This section explores Le Chatelier's Principle, explaining how equilibrium in a closed system reacts to changes in concentration, pressure, and temperature, ultimately shifting to a new equilibrium state. The principle applies to reversible reactions and highlights the dynamic nature of equilibrium.
Detailed
Le Chatelier's Principle
Le Chatelier's Principle states that if a system at dynamic equilibrium is subjected to a change in conditions (such as concentration, pressure, or temperature), the system will adjust itself in a way that partially counteracts the change, leading to a new equilibrium.
Key Features of Dynamic Equilibrium:
- Dynamic Nature: Reactions occur continuously in both directions at the equilibrium point.
- Constant Macroscopic Properties: Concentrations and other observable properties do not change over time.
- Closed Systems: Equilibrium is maintained only when the system is isolated from matter exchange with the surroundings.
- Reversible Reactions: Only reversible reactions can achieve equilibrium.
- Equilibrium Approach: The equilibrium can be arrived at from either direction of the reaction.
Predictions of Equilibrium Shifts:
- Concentration Changes:
- Adding/removing reactants or products influences the rate of the forward or reverse reactions.
- Pressure Changes:
- Significant only with gaseous reactions with different mole counts; increasing pressure favors the side with fewer moles.
- Temperature Changes:
- Affects both equilibrium position and the equilibrium constant; favors endothermic reactions at higher temperatures.
- Catalysts:
- Speed up the rate of reaching equilibrium but do not change the equilibrium position or constant.
Industrial Applications:
The Haber process for ammonia synthesis demonstrates these principles diversely, using adjustments in concentration, pressure, and temperature to optimize yields.
Youtube Videos

Audio Book
Dive deep into the subject with an immersive audiobook experience.
Introduction to Le Chatelier's Principle
Chapter 1 of 6
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
While equilibrium represents a stable state, it is not immutable. If conditions are changed, the equilibrium will shift. Le Chatelier's Principle provides a qualitative prediction for how an equilibrium system responds to a disturbance. It states:
If a system at dynamic equilibrium is subjected to a change in conditions, the system will adjust itself in a way that partially counteracts the change, thereby establishing a new equilibrium position.
Detailed Explanation
Le Chatelier's Principle tells us that systems at equilibrium can adjust to changes in their environment. If you alter a condition such as concentration, pressure, or temperature, the system will respond to counteract that change. For example, if you add more reactant, the equilibrium will shift to produce more product in order to restore balance.
Examples & Analogies
Think of a perfectly balanced seesaw with children on both sides. If one child gets off, the seesaw tilts. To regain balance, another child might need to get on the lighter side. Similarly, when conditions in a chemical equilibrium change, the reaction shifts to restore balance.
Understanding the Position of Equilibrium
Chapter 2 of 6
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
The 'position of equilibrium' refers to the relative amounts of reactants and products present at equilibrium. If the equilibrium shifts to the right (towards products), the concentration of products increases and reactants decreases. If it shifts to the left (towards reactants), the concentration of reactants increases and products decreases.
Detailed Explanation
The position of equilibrium indicates how much of each substance is present in the reaction mixture at equilibrium. A right shift means more products are formed, while a left shift indicates that more reactants are present. The equilibrium position depends on the specific conditions such as concentration and temperature.
Examples & Analogies
Imagine a cooking pot simmering on the stove. If you reduce the heat (cooling the pot), it may take longer for the food to cook (shift left towards reactants). If you increase the heat, the food cooks faster (shift right towards products). Just like cooking, chemical reactions adjust based on their conditions.
Effect of Concentration Changes
Chapter 3 of 6
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
- Effect of Concentration Changes:
- Adding a reactant (A or B): The system tries to consume the added reactant. The rate of the forward reaction increases, shifting the equilibrium to the right (towards products).
- Removing a reactant (A or B): The system tries to replenish the removed reactant. The rate of the reverse reaction increases (or forward rate decreases), shifting the equilibrium to the left (towards reactants).
- Adding a product (C or D): The system tries to consume the added product. The rate of the reverse reaction increases, shifting the equilibrium to the left (towards reactants).
- Removing a product (C or D): The system tries to replenish the removed product. The rate of the forward reaction increases, shifting the equilibrium to the right (towards products).
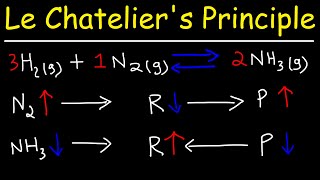
○ Industrial Application (Haber Process): In the synthesis of ammonia, N₂(g) + 3H₂(g) ⇌ 2NH₃(g), continuously removing the ammonia product (by liquefaction) shifts the equilibrium to the right, maximising the yield of ammonia.
Detailed Explanation
The concentration of reactants and products influences the direction of the equilibrium shift. When you add a reactant, the system shifts towards the products to offset this addition. Conversely, if a product is removed, the system shifts to produce more of that product. Understanding this helps in optimizing industrial processes, like the Haber Process for ammonia synthesis.
Examples & Analogies
Consider a restaurant's supply of ingredients. If they run low on tomatoes (a reactant), they may not be able to make enough spaghetti sauce (product). If they bring in more tomatoes, they'll quickly make more sauce. Similarly, in chemical reactions, adding or removing substances causes shifts to maintain balance.
Effect of Pressure Changes
Chapter 4 of 6
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
- Effect of Pressure Changes (for reactions involving gases):
- Pressure changes are only significant for reactions involving gases where there is a change in the total number of moles of gas. If the number of moles of gaseous reactants equals the number of moles of gaseous products (e.g., H₂(g) + I₂(g) ⇌ 2HI(g), where Δn_gas = 0), then pressure changes do not affect the position of equilibrium.
- Increasing the total pressure (by decreasing the volume of the container): The system attempts to reduce the pressure. It achieves this by favouring the side of the reaction with fewer moles of gas.
- Decreasing the total pressure (by increasing the volume of the container): The system attempts to increase the pressure. It achieves this by favouring the side of the reaction with more moles of gas.
○ Industrial Application (Haber Process): For N₂(g) + 3H₂(g) ⇌ 2NH₃(g), there are 4 moles of gaseous reactants and 2 moles of gaseous products. Therefore, increasing the pressure shifts the equilibrium to the right, increasing the yield of ammonia. Industrially, high pressures (e.g., 200 atm) are used.
Detailed Explanation
The pressure of a gaseous system affects equilibrium only when the number of gas molecules changes. Increasing pressure pushes the equilibrium towards the side with fewer gas moles to relieve the pressure, while decreasing pressure favors the side with more gas. This principle is widely applied in industrial processes to enhance product yield.
Examples & Analogies
Imagine inflating a balloon. If you squeeze the balloon (increase pressure), it tries to change shape. If you let it expand (decrease pressure), it fills up more. Similarly, in a reaction, increasing pressure forces it to yield more products if fewer gas moles are on that side.
Effect of Temperature Changes
Chapter 5 of 6
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
- Effect of Temperature Changes:
- Temperature changes are unique in that they affect both the position of equilibrium and the value of the equilibrium constant (K).
- Increasing the temperature: The system tries to absorb the added heat. This favours the endothermic reaction (the reaction that absorbs heat).
- Decreasing the temperature: The system tries to release heat. This favours the exothermic reaction (the reaction that releases heat).
○ If the forward reaction is exothermic (ΔH < 0), the reverse reaction is endothermic. Increasing temperature shifts equilibrium to the left; decreasing temperature shifts it to the right.
○ If the forward reaction is endothermic (ΔH > 0), the reverse reaction is exothermic. Increasing temperature shifts equilibrium to the right; decreasing temperature shifts it to the left.
○ Industrial Application (Haber Process): N₂(g) + 3H₂(g) ⇌ 2NH₃(g) is an exothermic reaction (ΔH = -92 kJ mol⁻¹). To maximise yield, a low temperature should be favoured. However, very low temperatures lead to very slow reaction rates. Therefore, a compromise temperature (around 400-450 °C) is used, which is high enough for a reasonable rate but low enough for a good equilibrium yield.
Detailed Explanation
Temperature changes can shift equilibrium either to favor the endothermic or exothermic reaction, depending on whether heat is added or removed. Endothermic reactions absorb heat, so increasing temperature shifts equilibrium in their favor, while exothermic reactions release heat and are favored when temperature decreases. This principle is vital in optimizing reaction conditions in industrial processes like the Haber Process.
Examples & Analogies
Think of a swimming pool. If it's particularly cold (low temperature), the water will feel even colder (exothermic favoring) if you add ice (shifting equilibrium). Conversely, if you bring in a heater, the pool temperature rises (endothermic favoring), causing the system to adjust and make the pool more comfortable. In chemicals, controlling temperature ensures the best reaction outcomes.
Effect of a Catalyst
Chapter 6 of 6
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
- Effect of a Catalyst:
- A catalyst is a substance that increases the rate of a chemical reaction without being consumed in the process.
- Crucially, a catalyst speeds up both the forward and reverse reactions by the same factor.
- Therefore, a catalyst helps the system reach equilibrium faster, but it does not change the position of equilibrium and does not affect the value of the equilibrium constant (K). It only affects the reaction rate.
○ Industrial Application (Haber Process): An iron-based catalyst is used to speed up the reaction, allowing equilibrium to be reached more quickly, without sacrificing the yield.
Detailed Explanation
Catalysts are essential in chemical reactions as they lower the activation energy required for the reaction to proceed, increasing rates without affecting the equilibrium position. This means that catalysts hasten the time it takes to reach equilibrium but do not shift the equilibrium itself. Their role is crucial in industrial processes for efficiency.
Examples & Analogies
Imagine baking cookies. If you preheat the oven (the catalyst), the cookies bake faster, but the number of cookies (equilibrium position) stays the same once they’re all finished. In a chemical reaction, a catalyst gets everyone working faster without changing the final outcome.
Key Concepts
-
Dynamic Equilibrium: A state where the rate of the forward reaction equals the rate of the reverse reaction.
-
Le Chatelier's Principle: Predicts how equilibrium shifts in response to changes in conditions.
-
Closed System: An isolated system where no reactants or products can escape.
-
Reversible Reaction: A reaction that can go in both forward and reverse directions.
Examples & Applications
In a reversible reaction where A ⇌ B, adding more A shifts the equilibrium towards B.
In the decomposition of N₂O₄ ⇌ 2NO₂, reducing the pressure favors the formation of more moles of N₂O₄.
Memory Aids
Interactive tools to help you remember key concepts
Rhymes
When temp goes up, endo takes a hand; shifting left, where cooling's planned.
Stories
Imagine a seesaw in a playground. When more kids sit on one side, the seesaw tips until it balances again. This represents how equilibrium shifts to regain balance.
Memory Tools
ACE: Add = Concentrate on products; helps remember how to shift equilibrium.
Acronyms
PCT
Pressure
Concentration
Temperature are key factors affecting equilibrium.
Flash Cards
Glossary
- Dynamic Equilibrium
The condition in a reversible reaction where the rates of the forward and reverse reactions are equal.
- Le Chatelier's Principle
A principle predicting how a system at equilibrium responds to changes in concentration, pressure, or temperature.
- Reversible Reaction
A reaction that can proceed in both the forward and reverse directions.
- Closed System
A physical system enclosed to prevent matter from entering or leaving.
- Endothermic Reaction
A reaction that absorbs heat from its surroundings.
- Exothermic Reaction
A reaction that releases heat into its surroundings.
Reference links
Supplementary resources to enhance your learning experience.
