Equilibrium Constant (Kc and Kp)
Enroll to start learning
You’ve not yet enrolled in this course. Please enroll for free to listen to audio lessons, classroom podcasts and take practice test.
Interactive Audio Lesson
Listen to a student-teacher conversation explaining the topic in a relatable way.
Understanding Kc
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Today, we're going to explore the equilibrium constant, Kc. Who can tell me what Kc represents?

I think Kc represents the ratio of concentrations at equilibrium.

That's correct! Kc is defined for a reaction like **aA + bB ⇌ cC + dD** as Kc = [C]ᶜ[D]ᶝ / [A]ᶦ[B]ᶦ. This means we look at the concentrations of products over reactants.

What do the brackets mean?

Great question! The brackets [ ] denote molar concentrations in mol/dm³. So it's how much of each substance is present at equilibrium.

Is Kc affected by anything?

Yes! Kc is temperature-dependent. If you change the temperature, Kc will change, but it remains constant at a specific temperature. That's really important to remember!

Can we use Kc for any reaction?

Not for every reaction! Only for reversible reactions in a closed system. Well done, everyone! Today, we learned that Kc helps us understand how far a reaction goes to completion.
Kp: The Partial Pressure Constant
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Now, let's talk about Kp. How does it differ from Kc?

Is Kp just for gases?

Exactly! Kp is used when we express the equilibrium in terms of partial pressures of gases. For the reaction, Kp = (PA)ᶦ(PB)ᶦ(PC)ᶜ(PD)ᶝ.

What are partial pressures?

Partial pressure is the pressure a gas would exert if it occupied the volume by itself. It’s a useful way to analyze gaseous reactions.

How are Kp and Kc related?

Great question! The relationship is Kp = Kc(RT)ᵈΔn_gas, where Δn_gas indicates the change in moles of gas. This equation connects the two constants!

Are there situations where Kp and Kc are equal?

Yes, when Δn_gas = 0, Kp equals Kc. Excellent participation! Today we've extended our knowledge to include Kp and its significance in gas reactions.
Significance of K Magnitudes
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

What does it mean if K is much greater than 1?

It means the reaction goes to completion, right?

Exactly! A large K value means that products are favored at equilibrium. Conversely, what about if K is much smaller than 1?

Then the reactants are favored.

Correct again! A small K indicates that very little product has formed. If K is close to 1, what can we infer?

Both reactants and products are present in significant amounts!

Absolutely! Understanding the magnitude of K allows us to predict the extent of a reaction. Well done, team!
Introduction & Overview
Read summaries of the section's main ideas at different levels of detail.
Quick Overview
Standard
The section explains the definitions and formulations of the equilibrium constants (Kc and Kp) for reversible reactions. It highlights the significance of temperature in determining Kc, the inclusion of phases in expressions, and the relationship between Kc and Kp through the change in moles of gas (Δngas). It also details the implications of the magnitude of K in relation to the extent of a reaction.
Detailed
Detailed Summary of Equilibrium Constant (Kc and Kp)
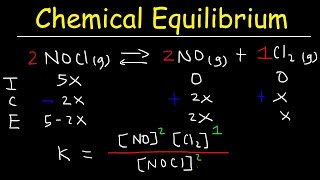
In chemical equilibrium, the equilibrium constant (K) offers a quantitative measure of a reaction's extent at a given temperature. For a reversible reaction:
aA + bB ⇌ cC + dD
The equilibrium constant in terms of concentrations, denoted as Kc, is defined by the formula:
Kc = [C]ᶜ[D]ᶝ / [A]ᶦ[B]ᶦ
Where:
- [A], [B], [C], [D] represent the molar concentrations of the respective reactants and products, expressed in mol/dm³.
- a, b, c, d are their respective stoichiometric coefficients from the balanced chemical equation.
- The square brackets indicate molar concentration.
Important Points About Kc
- Temperature Dependence: Kc varies with temperature changes; it is constant only at a specific temperature.
- Phases of Matter: Kc expressions only include gaseous and aqueous reactants/products; solids and liquids, having constant concentrations, are excluded.
- Example: For the reaction CaCO₃(s) ⇌ CaO(s) + CO₂(g), the Kc expression is simply Kc = [CO₂].
- Magnitude of Kc:
- K >> 1 implies the equilibrium lies to the right (products favored).
- K << 1 implies it lies to the left (reactants favored).
- K near 1 indicates significant amounts of both reactants and products.
For reactions involving gases, Kp provides an alternative expression based on partial pressures, defined as:
Kp = (Pᴬ)ᶦ(Pᴮ)ᶦ(Pᶜ)ᶜ(Pᶝ)ᶝ
Where P represents the partial pressures.
Relationship Between Kp and Kc
The relationship is given by:
Kp = Kc (RT)ᵈΔnᴳᵃˢ
Where Δn_gas is the change in total moles of gas during the reaction.
If Δn_gas = 0, then Kp = Kc.
This section emphasizes the critical evaluation of reaction extents, making it foundational for understanding both the quantitative analysis of dynamic equilibrium and the development of practical applications in chemical processes.
Youtube Videos

Audio Book
Dive deep into the subject with an immersive audiobook experience.
Definition of Equilibrium Constant (Kc)
Chapter 1 of 4
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
While Le Chatelier's Principle helps predict the direction of an equilibrium shift, the equilibrium constant (K) provides a quantitative measure of the extent to which a reaction proceeds to completion at a specific temperature. It expresses the ratio of product concentrations (or partial pressures) to reactant concentrations (or partial pressures) at equilibrium.
For a general reversible reaction at equilibrium:
aA + bB ⇌ cC + dD
The equilibrium constant in terms of concentrations (Kc) is defined as:
Kc =[A]a[B]b[C]c[D]d
Where:
● [A], [B], [C], [D] represent the equilibrium molar concentrations of the respective reactants and products, expressed in mol dm⁻³.
● a, b, c, d are the stoichiometric coefficients from the balanced chemical equation.
● The square brackets ([ ]) denote molar concentration.
Detailed Explanation
The equilibrium constant, Kc, quantifies how far a reaction proceeds towards forming products at equilibrium. It is defined based on the concentrations of products and reactants at that point. This formula allows us to calculate Kc using the concentrations of the substances involved in a reaction. For instance, in the equation Kc = [C]c[D]d / [A]a[B]b:
- The reactants A and B have their concentrations raised to the power of their coefficients in the balanced equation, and similarly for the products C and D. This ratio provides a specific numeric value that reflects the extent of the reaction under given conditions.
Examples & Analogies
Think of Kc as a scoreboard in a game between reactants and products. The scores (concentrations) change throughout the game (reaction) but at the end, the Kc value tells you who won and by how much. A high Kc means products dominated the game, while a low Kc suggests reactants held control.
Important Considerations for Kc
Chapter 2 of 4
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Important Considerations for Kc:
● Temperature Dependence: The value of Kc is constant for a given reaction only at a specific temperature. If the temperature changes, the value of Kc will change. This is the only factor that alters the numerical value of K.
● Inclusion of Phases: Only species that have variable concentrations (gases and aqueous solutions) are included in the Kc expression. Pure solids and pure liquids have constant concentrations (their amount per unit volume is effectively constant at a given temperature and pressure) and are therefore omitted from the Kc expression.
Example: For the reaction CaCO₃(s) ⇌ CaO(s) + CO₂(g), the Kc expression is simply Kc = [CO₂].
● Units of Kc: The units of Kc depend on the stoichiometry of the reaction. While technically Kc has units (e.g., mol dm⁻³), it is often treated as dimensionless in IB Chemistry calculations because the rigorous definition of K involves "activities" rather than true concentrations, making it dimensionless. However, you should be aware that the units can be derived.
● Magnitude of Kc and Extent of Reaction: If K is very large (K >> 1): The equilibrium lies far to the right... If K is very small (K << 1): The equilibrium lies far to the left... If K is close to 1: Significant amounts of both reactants and products are present at equilibrium.
Detailed Explanation
Here are some key factors to understand about Kc:
1. Temperature Dependence: Kc is only valid at a specific temperature; changing temperature alters its value.
2. Inclusion of Phases: Only gases and aqueous solutions are counted in the Kc calculation, while pure solids and liquids are excluded because their concentrations do not change significantly.
3. Units: Kc can have units but is often treated as dimensionless for simplicity.
4. Magnitude: A large Kc means that products are favored at equilibrium, while a small Kc indicates reactants dominate.
Examples & Analogies
Imagine having a recipe that changes based on temperature - if you bake a cake at a higher heat, the cake might rise too much (like having a large Kc), but if it’s too low, it won’t rise at all (like a small Kc). Understanding how temperature affects your cake helps clarify how Kc tells you about the conditions under which a reaction occurs.
Equilibrium Constant in terms of Partial Pressures (Kp)
Chapter 3 of 4
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Equilibrium Constant in terms of Partial Pressures (Kp):
For reactions involving gases, it is often more convenient to express concentrations in terms of partial pressures. The equilibrium constant in terms of partial pressures (Kp) is defined similarly to Kc:
Kp =(PA )a(PB )b(PC )c(PD )d
Where:
● P_A, P_B, P_C, P_D represent the equilibrium partial pressures of the respective gaseous reactants and products. Partial pressure is the pressure that a gas would exert if it alone occupied the volume of the mixture at the same temperature.
● The exponents (a, b, c, d) are again the stoichiometric coefficients.
Detailed Explanation
Kp is another way to express the equilibrium constant but focuses on partial pressures instead of concentrations. This is particularly useful for gas reactions. The formula is similar to Kc, with pressures replacing concentrations. It provides the same insights into how a reaction favors products or reactants but is more applicable when dealing with gaseous substances.
Examples & Analogies
Think of Kp as measuring the pressure in a balloon filled with different gases. The overall pressure can tell you about the balance of gases inside the balloon, just like Kp tells you about the balance of partial pressures of gases in a chemical reaction.
Relationship between Kc and Kp
Chapter 4 of 4
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Relationship between Kc and Kp:
Kp and Kc are related by the following equation:
Kp = Kc (RT)^Δn_gas
Where:
● R is the ideal gas constant (8.314 J K−1 mol−1 or 0.08206 L atm mol−1 K−1; choose units consistent with pressure).
● T is the absolute temperature in Kelvin (K).
● Δn_gas is the change in the total number of moles of gas during the reaction: Δn_gas = (sum of stoichiometric coefficients of gaseous products) - (sum of stoichiometric coefficients of gaseous reactants).
If Δn_gas = 0 (i.e., the total moles of gaseous reactants equals the total moles of gaseous products), then (RT)^0 = 1, and therefore Kp = Kc.
Detailed Explanation
Kc and Kp are closely linked through an equation that accounts for the change in the number of gas moles during a reaction. This relation helps convert between the two forms of the equilibrium constant if you know the temperature and the changes in gas moles. If the number of reactant and product gas moles is equal, Kp and Kc will have the same value.
Examples & Analogies
Consider measuring the speed of a car in both mph and km/h; they represent the same speed but in different units. Kc and Kp work similarly - they offer different perspectives (molar concentration vs. pressure) on the same equilibrium situation.
Key Concepts
-
Kc Definition: The equilibrium constant based on concentration, varying with temperature.
-
Kp Definition: The equilibrium constant based on partial pressure, applicable to gaseous reactions.
-
Magnitude Interpretation: High K favors products; low K favors reactants.
-
Relationship Between Kc and Kp: Kp relates to Kc via the formula Kp = Kc(RT)ᵈΔn_gas.
Examples & Applications
For the reaction N₂(g) + 3H₂(g) ⇌ 2NH₃(g), Kc can be calculated using equilibrium concentrations.
In a reaction where Kp = 0.8 and Δn_gas = -1, Kc can be found using Kp = Kc(RT)ᵈΔn_gas if R and T are known.
Memory Aids
Interactive tools to help you remember key concepts
Rhymes
Kc shows us in equilibrium, how products line up in the room.
Stories
Imagine a garden where flowers (products) grow more dense than weeds (reactants); the garden is balanced at Kc.
Memory Tools
Kc Constant Clouds: Concentration Counts when calculating K.
Acronyms
Kc = Cool Concentration
Products vs. Reactants.
Flash Cards
Glossary
- Equilibrium Constant (Kc)
A constant that quantifies the ratio of product concentrations to reactant concentrations at equilibrium for reversible reactions.
- Equilibrium Constant (Kp)
An expression that quantifies the ratio of partial pressures of products to reactants at equilibrium for reactions involving gases.
- Molar Concentration
The amount of a substance (in moles) divided by the volume of the solution (in dm³), expressed as mol/dm³.
- Partial Pressure
The pressure a gas would exert if it occupied the entire volume of the mixture alone.
- Δn_gas
The change in the total number of moles of gas during a chemical reaction.
Reference links
Supplementary resources to enhance your learning experience.
