Concentration of Ore (Ore Dressing)
Enroll to start learning
You’ve not yet enrolled in this course. Please enroll for free to listen to audio lessons, classroom podcasts and take practice test.
Interactive Audio Lesson
Listen to a student-teacher conversation explaining the topic in a relatable way.
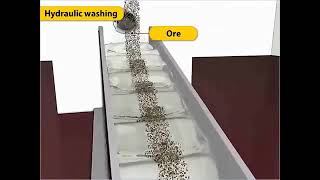
Hydraulic Washing
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Today, we are going to learn about hydraulic washing. This method involves using water to separate impurities from heavier ores. Can anyone tell me what this method achieves?

This helps in separating lighter gangue from the heavier ores.

Exactly! By allowing water to wash away the lighter materials, we can concentrate the heavy valuable ores. This is particularly useful in ores like iron. What's a good way to remember this method?

Maybe something like, 'Wash your ores just like your car!'?

That's a fun way to put it! Remember that hydraulic washing helps in retrieving ores efficiently. Let’s summarize this point: hydraulic washing uses water to wash away lighter impurities for ore concentration.
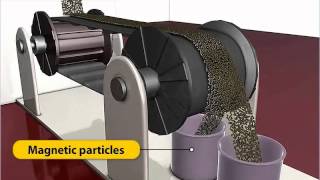
Magnetic Separation
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Next, let's discuss magnetic separation. Can someone explain why we use magnets in this method?

We use magnets to attract magnetic ores, like magnetite!

Right! This method is particularly useful for materials that contain iron. It helps in quickly separating the valuable resources from gangue particles. How might we remember this process?

Maybe with a phrase like, 'Magnet to the rescue!' or something similar?

Fantastic! So, magnetic separation can be summed up as using magnets to separate magnetic ores from non-magnetic gangue effectively.
Froth Flotation
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

In this session, we'll dive into froth flotation. This process is crucial for sulfide ores. What happens to the ores in this method?

The sulfide ores float on top, while the gangue sinks!

Exactly! It’s fascinating how we can separate materials this way. Can anyone suggest a simple mnemonic to remember this method?

How about 'Floats like a boat, sinks like a stone' to emphasize separation?

That’s perfect! Thus, in froth flotation, sulfide ores float while the gangue sinks, allowing easy separation.
Leaching
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Finally, we're going to discuss leaching. This is a technique where we dissolve ores in a solvent. Can someone share an example?

Bauxite is leached using sodium hydroxide!

Excellent! Through leaching, we can extract aluminum from bauxite efficiently. How about a memory aid for this method?

Maybe 'Leach your worries away!' to denote the process of dissolving impurities!

Great! In summary, leaching involves dissolving the ore in a solvent to extract valuable metal, exemplified by bauxite leaching in NaOH.
Introduction & Overview
Read summaries of the section's main ideas at different levels of detail.
Quick Overview
Standard
This section discusses the various methods of ore concentration, also known as ore dressing, which are critical for separating valuable minerals from unwanted materials. Techniques such as hydraulic washing, magnetic separation, froth flotation, and leaching are highlighted, each serving a unique purpose in the ore processing industry.
Detailed
Detailed Summary
Concentration of Ore (Ore Dressing)
Concentration of ore is a fundamental step in the metal extraction process, where valuable minerals are separated from waste materials known as gangue. This section highlights four primary methods:
- Hydraulic Washing: This technique utilizes water to differentiate between heavy ores and lighter impurities, allowing for a
Youtube Videos








Audio Book
Dive deep into the subject with an immersive audiobook experience.
Hydraulic Washing
Chapter 1 of 4
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
● Hydraulic Washing: Using water to wash away lighter impurities.
Detailed Explanation
Hydraulic washing is a method used to separate ores from lighter impurities by utilizing the force of water. The heavier ore sinks while the lighter impurities are washed away with the water, allowing for a cleaner concentrated ore. This method is particularly useful for materials that are not heavily bound with impurities.
Examples & Analogies
Imagine trying to separate sand from small pebbles. If you pour water over them, the sand, being lighter, gets washed away while the heavier pebbles remain at the bottom. This is essentially what hydraulic washing achieves with ore and its impurities.
Magnetic Separation
Chapter 2 of 4
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
● Magnetic Separation: Using magnets to separate magnetic ores (e.g., Fe₃O₄).
Detailed Explanation
Magnetic separation is a technique used to separate magnetic materials from non-magnetic materials. In this process, strong magnets are employed to attract magnetic ores, such as magnetite (Fe₃O₄). This allows for the efficient separation of the desired metallic ore from the gangue, facilitating further processing.
Examples & Analogies
Think of how a refrigerator magnet sticks to the fridge. In magnetic separation, we use similar magnets to pull out metallic ores from a mixture, almost like fishing for fish with a magnet instead of a hook.
Froth Flotation
Chapter 3 of 4
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
● Froth Flotation: For sulfide ores (e.g., ZnS, PbS), where ores float while gangue sinks.
Detailed Explanation
Froth flotation is a separation process that relies on the differences in the hydrophobic properties of ore particles. This technique is especially useful for sulfide ores. In the flotation tank, air is bubbled through a slurry of ground ore and water, causing the sulfide minerals to attach to the bubbles and rise to the surface as froth, while the gangue sinks. The froth can then be skimmed off for further processing.
Examples & Analogies
Imagine adding bubbles to a bath; some toys (like rubber ducks) float while heavier ones sink. Froth flotation uses this principle to separate valuable minerals from waste by getting them to float on the surface.
Leaching
Chapter 4 of 4
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
● Leaching: Dissolving ore in a suitable solvent (e.g., bauxite in NaOH).
Detailed Explanation
Leaching is a chemical process by which metals are dissolved from their ores in a suitable solvent. This method is effective for ores that might not be separable through mechanical methods. For instance, bauxite, which contains aluminum, can be treated with sodium hydroxide (NaOH) to dissolve the aluminum, leaving behind other materials. The aluminum can then be precipitated out for extraction.
Examples & Analogies
Imagine making sweet tea by adding sugar to hot water. The sugar dissolves, allowing you to enjoy the sweet flavor later. In leaching, the metal dissolves in the solvent, similar to how sugar dissolves in water.
Key Concepts
-
Hydraulic Washing: A separation technique using water to remove impurities from heavier ores.
-
Magnetic Separation: Using magnets to attract and separate magnetic ores from non-magnetic materials.
-
Froth Flotation: Separating sulfide ores by making them float while non-valuable materials sink.
-
Leaching: Dissolving ores in solvents to efficiently extract metals.
Examples & Applications
In hydraulic washing, iron ore can be concentrated by washing away lighter impurities like sand and clay.
In magnetic separation, magnetite can be isolated from non-magnetic gangue material.
In froth flotation, zinc sulfide ores are separated from pyrite where the former floats and the latter sinks.
Leaching is used to extract aluminum from bauxite through dissolution in sodium hydroxide.
Memory Aids
Interactive tools to help you remember key concepts
Rhymes
If your ore is too light, wash it with might, get rid of the dirt to extract what’s right!
Stories
Imagine a precious metal hidden in dirt. A wise miner uses water to wash away the dirt and reveal a shiny gem. That's hydraulic washing in action!
Memory Tools
Remember: 'W.M.F.L' - Water for hydraulic washing, Magnets for magnetic separation, Froth for froth flotation, Liquids for leaching!
Acronyms
For remembering the methods
'HMF' - Hydraulic Washing
Magnetic Separation
Froth Flotation!
Flash Cards
Glossary
- Hydraulic Washing
A method of ore concentration using water to separate lighter impurities from heavier ores.
- Magnetic Separation
A process that uses magnets to separate magnetic ores from non-magnetic gangue.
- Froth Flotation
A process for separating sulfide ores where the ores float in froth while gangue sinks.
- Leaching
The process of dissolving ore in a suitable solvent to extract metals.
- Gangue
Unwanted material found in ores, often consisting of sand, clay, or other impurities.
Reference links
Supplementary resources to enhance your learning experience.
