Reduction of Oxides to Metals
Enroll to start learning
You’ve not yet enrolled in this course. Please enroll for free to listen to audio lessons, classroom podcasts and take practice test.
Interactive Audio Lesson
Listen to a student-teacher conversation explaining the topic in a relatable way.
Reduction Methods Overview
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Today, we're going to learn about the reduction of metal oxides to obtain pure metals. Can anyone tell me what reduction means in this context?

Isn't reduction when we remove oxygen from metal ores?

Exactly, Student_1! We reduce metal oxides, meaning we remove oxygen to obtain the metal. There are several methods to do this, including using carbon or carbon monoxide. Who wants to start with carbon reduction?
Reduction by Carbon
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Reduction by carbon is a common method. For example, zinc oxide reacts with carbon. Can someone share the reaction equation?

It's ZnO + C → Zn + CO!

Great job, Student_2! This equation shows that zinc oxide is reduced to zinc while carbon is oxidized to carbon monoxide. Why do you think carbon is often used?

Because it’s readily available and cheap?

Exactly! Cost-effectiveness is a significant reason.
Reduction by Carbon Monoxide
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Now let's talk about carbon monoxide. When it reacts with iron(III) oxide, what do we produce?

Iron and carbon dioxide?

Perfect! The reaction is Fe₂O₃ + 3CO → 2Fe + 3CO₂. Carbon monoxide is also effective due to its ability to act as a reducing agent at lower temperatures than carbon. Why is this important?

It can prevent unwanted reactions with the metal?

Exactly! It’s crucial in metallurgy to avoid complications during metal extraction.
Electrolysis
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

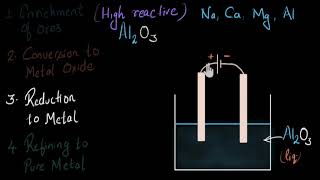
Lastly, we have electrolysis. This method is particularly important for very reactive metals. Who can name one example?

Aluminum!

Exactly! We use electrolysis for aluminum extraction from its ore. This method involves dissolving alumina in cryolite and using electrical current. Why do you think we use electrolysis instead of carbon here?

Because aluminum is too reactive to be reduced by carbon?

Right! Great reasoning. This knowledge will prove vital as we continue.
Summary of Reduction Methods
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Let's recap our discussion. What are the three methods we covered for reducing metal oxides?

Reduction by carbon, carbon monoxide, and electrolysis!

Excellent! Each method has its significance based on the metal's reactivity. Remember this: 'C for Carbon, CO for Carbon Monoxide, and Electrolysis for Energetic.' This can help you recall the reduction methods.
Introduction & Overview
Read summaries of the section's main ideas at different levels of detail.
Quick Overview
Standard
The reduction of metal oxides is crucial in metallurgy for extracting metals from their ores. This section discusses three primary methods: reducing with carbon, using carbon monoxide, and employing electrolysis for very reactive metals. Each method plays a role depending on the reactivity of the metals being extracted.
Detailed
Reduction of Oxides to Metals
In metallurgy, the reduction of metal oxides is essential for extracting metals from ores. This section outlines three primary reduction methods:
- Reduction by Carbon: In this method, carbon is used to reduce metal oxides. For example, zinc oxide (ZnO) reacts with carbon (C) to produce zinc (Zn) and carbon monoxide (CO):
Equation: ZnO + C → Zn + CO
- Reduction by Carbon Monoxide: Another technique utilizes carbon monoxide (CO) to reduce metal oxides. For example, iron(III) oxide (Fe₂O₃) can be reduced with carbon monoxide to yield iron (Fe) and carbon dioxide (CO₂):
Equation: Fe₂O₃ + 3CO → 2Fe + 3CO₂
- Reduction by Electrolysis: For very reactive metals like aluminum (Al) and sodium (Na), electrolysis is employed, where the metal is separated using electrical energy rather than chemical reductants.
Understanding these methods is vital since the choice of a reduction method can significantly influence the efficiency and cost of metal extraction processes.
Youtube Videos






Audio Book
Dive deep into the subject with an immersive audiobook experience.
Reduction by Carbon
Chapter 1 of 3
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
● By Carbon: ZnO + C → Zn + CO
Detailed Explanation
In the reduction of metal oxides, carbon is often used as a reducing agent. The reaction involves zinc oxide (ZnO) reacting with carbon (C) to produce zinc (Zn) and carbon monoxide (CO). This process takes place at high temperatures, where carbon donates electrons to ZnO, effectively removing the oxygen and resulting in the formation of metallic zinc.
Examples & Analogies
Think of carbon as a powerful helper that assists in 'freeing' zinc from its oxide jail. Just as a helper might assist someone to escape wrongful imprisonment by providing an exit route, carbon helps remove the attached oxygen from zinc oxide, allowing pure zinc to emerge.
Reduction by Carbon Monoxide
Chapter 2 of 3
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
● By Carbon Monoxide: Fe₂O₃ + 3CO → 2Fe + 3CO₂
Detailed Explanation
Another common method for reduction is using carbon monoxide (CO). In this process, iron(III) oxide (Fe₂O₃) reacts with carbon monoxide to produce iron (Fe) and carbon dioxide (CO₂). The CO acts as a reducing agent, removing oxygen from the iron oxide, thereby converting it into metallic iron. This method is particularly effective at temperatures in the range typically found in industrial furnaces.
Examples & Analogies
Imagine a situation where carbon monoxide is like a strategic negotiator, persuading the iron oxide (Fe₂O₃) to give up its oxygen. Just like a negotiator helps settle disputes by getting the parties to agree on a solution, CO resolves the bond between iron and oxygen, allowing iron to stand on its own as a pure metal.
Reduction by Electrolysis
Chapter 3 of 3
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
● By Electrolysis: Used for reactive metals like Al, Na, etc.
Detailed Explanation
For the extraction of highly reactive metals such as aluminum (Al) and sodium (Na), electrolysis is the preferred method. In this process, an electric current is passed through a molten or dissolved form of the metal's compound. This leads to the breakdown of the compound into its elemental form. During electrolysis, positive metal ions move towards the cathode (negative electrode), where they gain electrons and become neutral metal atoms.
Examples & Analogies
Picture electrolysis as a high-tech assembly line, where electricity serves as the catalyst that splits the compound apart. Just like factory workers might break down materials into their components to create something new, electrolysis uses electrical energy to separate metal from its compound, allowing metals like aluminum to be produced in a pure form.
Key Concepts
-
Reduction by Carbon: The method of using carbon to remove oxygen from metal oxides.
-
Reduction by Carbon Monoxide: A process that uses CO as a reducing agent at lower temperatures.
-
Electrolysis: A method for extracting highly reactive metals using electrical energy.
Examples & Applications
Zinc extraction from zinc oxide using carbon: ZnO + C → Zn + CO.
Iron extraction from iron(III) oxide using carbon monoxide: Fe₂O₃ + 3CO → 2Fe + 3CO₂.
Electrolysis used for aluminum extraction from its ore.
Memory Aids
Interactive tools to help you remember key concepts
Rhymes
Carbon's a slab, reducing zinc from the lab.
Stories
Once upon a time, in a chemistry lab, there was a brave Zinc who wanted to escape the grasp of his oxide prison. He called for Carbon, and through great heat, he was freed!
Memory Tools
Remember: C for Carbon, CO for Carbon Monoxide, Electrolysis for Energetic extraction!
Acronyms
RCE
Reduction by Carbon
by CO
and by Electrolysis.
Flash Cards
Glossary
- Reduction
The process of removing oxygen from a compound, often to obtain a pure metal from its oxide.
- Metal Oxides
Compounds consisting of metal and oxygen that require reduction to extract pure metals.
- Carbon Reduction
A method of reducing metal oxides using carbon as a reductant.
- Electrolysis
A technique that uses electrical energy to drive a non-spontaneous chemical reaction for metal extraction.
Reference links
Supplementary resources to enhance your learning experience.
