Anomalous Properties of Second Period Elements
Enroll to start learning
You’ve not yet enrolled in this course. Please enroll for free to listen to audio lessons, classroom podcasts and take practice test.
Interactive Audio Lesson
Listen to a student-teacher conversation explaining the topic in a relatable way.
Introduction to Anomalous Properties
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Welcome, everyone! Today we're looking at the anomalous properties of second-period elements, specifically lithium and beryllium. Can anyone tell me what 'anomalous' means?

Is it when something is different from the usual?

Exactly! These elements are different from others in their group. For example, while alkali metals typically form ionic compounds, lithium tends to form covalent ones due to its small size. Can anyone explain why smaller size affects bond formation?

Because smaller atoms can get closer to other atoms, so the bond can be stronger?

Correct! The closer proximity enables stronger interactions. Now, let's remember this with the acronym 'LBC' for 'Lithium Bonds Covalently.'
Behavior of Beryllium vs Other Group Elements
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Next, let's talk about beryllium. How does its behavior contrast with that of other alkaline earth metals?

Beryllium forms covalent compounds instead of ionic ones, right?

Yes! That's because its charge-to-radius ratio is high. What does that mean in terms of electronegativity?

It means it has a strong attraction for electrons.

Exactly! This is a crucial point. To help us remember high electronegativity, let's use 'Be a High E,' where 'E' represents electronegativity.
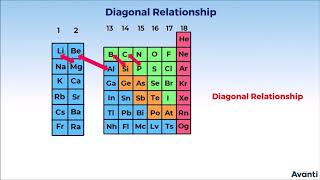
Diagonal Relationships
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Now, let's discuss the concept of diagonal relationship. Why might Li and Be resemble Mg and Al?

Because they share similar properties despite being in different groups?

Exactly! Li behaves similarly to Mg and Be to Al. This is due to their charge-to-radius ratio. Can you think of another factor contributing to these similarities?

The size and electronegativity?

Very good! Their smaller size increases their charge density, leading to differences in their ionic and covalent bonding tendencies.
Bonding Capabilities
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Let's move on to bonding capabilities. Why do some elements have a maximum covalency of four?

Because they only have four orbitals to share electrons, unlike bigger elements that can use d-orbitals too?

Exactly! This limitation allows for a distinctive behavior in covalent structures, especially in compounds of these elements. How can we remember this point?

Maybe something like 'Maximum Four' as a reminder?

Great idea! 'Max Four' will work well as a mnemonic to help keep that in mind.
Summary of Key Concepts
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

To summarize, today we discussed the anomalous properties of Li and Be, how they form covalent rather than ionic compounds, their diagonal relationships with Mg and Al, and the limitations in maximum covalency. Can someone summarize why these properties are significant?

They help us understand periodic trends and how size and electronegativity influence bonding!

Perfect! If you keep these ideas in mind, you will see how they align with the structure of the periodic table.
Introduction & Overview
Read summaries of the section's main ideas at different levels of detail.
Quick Overview
Standard
The section details the anomalous properties of second-period elements, focusing on how lithium and beryllium differ from other alkali and alkaline earth metals in forming covalent compounds. Additionally, it discusses the diagonal relationship between these elements and their subsequent group members, explaining why their behaviors diverge due to factors like small atomic size and high electronegativity.
Detailed
Anomalous Properties of Second Period Elements
The anomalous properties of the first member of each group from lithium (Li) in Group 1 to fluorine (F) in Group 17 are key to understanding periodic trends. These elements often exhibit distinct characteristics compared to their heavier counterparts. For instance:
- Lithium and Beryllium: While other alkali metals typically form ionic compounds, lithium demonstrates a strong tendency to form covalent bonds due to its relatively small size and electronegativity. Similarly, beryllium, unlike other alkaline earth metals, exhibits pronounced covalent behavior. This difference arises because both lithium and beryllium have higher charge-to-radius ratios, leading to stronger interactions with other atoms.
- Chemical Behavior: The first elements of groups (Li and Be) can be likened in their behavior to magnesium (Mg) and aluminum (Al) respectively, showcasing diagonal relationships in the periodic table. The greater charge-to-radius ratio in Li and Be enables high electronegativity, affecting their bonding nature.
- Bonding Capabilities: The first member of each p-block group is also limited in bonding capacities to a maximum covalency of four, contrasting with other members that can utilize additional d-orbitals for bonding, resulting in diverse oxidation states and complex compounds.
In summary, the unique properties of these elements indicate critical periodic trends and reinforce the concept of the periodic table's structure as it relates to chemical reactivity and bonding.
Youtube Videos






Audio Book
Dive deep into the subject with an immersive audiobook experience.
Characteristics of Lithium and Beryllium
Chapter 1 of 4
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
The first element of each of the groups 1 (lithium) and 2 (beryllium) and groups 13-17 (boron to fluorine) differs in many respects from the other members of their respective group. For example, lithium unlike other alkali metals, and beryllium unlike other alkaline earth metals, form compounds with pronounced covalent character; the other members of these groups predominantly form ionic compounds.
Detailed Explanation
In the periodic table, lithium (Li) is the first alkali metal and beryllium (Be) is the first alkaline earth metal. These elements have unique properties compared to their group members. While most alkali metals (like sodium and potassium) form ionic bonds easily with nonmetals, lithium forms covalent bonds due to its smaller size and higher electronegativity. Similarly, beryllium, unlike other alkaline earth metals which typically form ionic compounds, also forms covalent compounds due to its relatively small size and high charge density.
Examples & Analogies
Think of lithium and beryllium as two individuals who excel in a unique way within a group. In a basketball team, for instance, while most players are tall and excel at slam dunks (like sodium or magnesium), a shorter player like Li might excel at three-point shots (forming covalent bonds), and a more stocky player like Be might play strategically, making calculated passes (forming covalent compounds).
Similarities with Second Elements
Chapter 2 of 4
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
In fact, the behaviour of lithium and beryllium is more similar with the second element of the following group i.e., magnesium and aluminium, respectively.
Detailed Explanation
The behavior of lithium and beryllium shows notable similarities to magnesium and aluminum, respectively. This is due to the diagonal relationship in the periodic table, where elements can display similar traits despite being in different groups. For example, both Mg and Be can form covalent bonds and have similar types of compounds, showcasing a blend of properties that are characteristic of both groups.
Examples & Analogies
Imagine lithium and beryllium as being more like their diagonal friends in school, magnesium and aluminum. They share similar interests in sports (covalent bond formation) and thus, instead of sticking solely to their own teams (ionic compounds), they often collaborate together, showcasing that sometimes friendships cross boundaries (different groups).
Reasons for Anomalous Behavior
Chapter 3 of 4
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
The anomalous behaviour is attributed to their small size, large charge/radius ratio and high electronegativity of the elements.
Detailed Explanation
The unique behavior of lithium and beryllium can largely be explained by their small atomic sizes and high charge-to-radius ratios. Because they are small, they have a stronger pull on their bonding electrons, making them more likely to form covalent bonds rather than ionic ones. Additionally, their high electronegativities mean they have a strong tendency to attract electrons, which further influences their bonding characteristics.
Examples & Analogies
Think of these elements like two tiny magnets. Due to their small size (strong attraction), they can grip their partners (electrons) more tightly compared to larger magnets (the other alkali or alkaline earth metals), which makes them very effective at forming specific bonds and exhibiting unique behaviors.
Valence Electron Configuration and Covalency Limits
Chapter 4 of 4
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
In addition, the first member of each group has only four valence orbitals (2s and 2p) available for bonding, whereas the second member of the groups have nine valence orbitals (3s, 3p, 3d). As a consequence of this, the maximum covalency of the first member of each group is 4.
Detailed Explanation
Lithium and beryllium, being the first members of their respective groups, have fewer orbitals available for bonding (only 2s and 2p). This limits their ability to form multiple bonds compared to their successors, who have access to additional orbitals (including d-orbitals) allowing for greater versatility in bonding. Consequently, elements like boron (Group 13) can form up to four covalent bonds, but other group members can expand beyond that due to their additional orbitals.
Examples & Analogies
Imagine you're at a dance party. Lithium and beryllium are like guests who can only dance with a maximum of four partners because they have limited space on the dance floor. In contrast, magnesium and aluminum can dance with more partners because they have a larger area to move around (more orbitals). This limited capacity shapes how they interact with others, reinforcing their unique roles in the group.
Key Concepts
-
Lithium and Beryllium's Unique Behavior: They form covalent rather than ionic compounds.
-
Diagonal Relationships: Similar properties with magnesium and aluminum due to charge-to-radius ratios.
-
Maximum Covalency: Li and Be demonstrate a limit in covalent bonding compared to heavier group members.
Examples & Applications
Lithium (Li) forms compounds like LiCl where covalent behavior is observed.
Beryllium (Be) shows covalent characteristics in compounds, differing from typical alkaline earth metals.
Memory Aids
Interactive tools to help you remember key concepts
Rhymes
Li and Be, bonds like glue, form covalent streams, that’s their queue.
Stories
Once in a lab, Lithium and Beryllium looked at their friends. They noticed while others had ionic bonds, they preferred to stay together sharing their electrons, creating strong covalent friendships!
Memory Tools
Remember 'LiBe' for 'Lithium Beryllium' in covalent bonding scenarios!
Acronyms
Use 'CBE' which stands for 'Covalent bonding of Beryllium and Electronegativity' to recall their unique behavior.
Flash Cards
Glossary
- Anomalous Properties
Characteristics that differ significantly from the common behavior of elements in a group.
- Covalent Bonding
A type of chemical bond where electrons are shared between atoms.
- ChargetoRadius Ratio
The ratio of the positive charge of the nucleus to the atomic or ionic size, influencing the element's reactivity.
- Diagonal Relationship
The similarity in properties of elements in different groups that are diagonal to each other in the periodic table.
- Maximum Covalency
The maximum number of covalent bonds an element can form, often limited by its valence orbitals.
Reference links
Supplementary resources to enhance your learning experience.
