Ionization Enthalpy
Enroll to start learning
You’ve not yet enrolled in this course. Please enroll for free to listen to audio lessons, classroom podcasts and take practice test.
Interactive Audio Lesson
Listen to a student-teacher conversation explaining the topic in a relatable way.
What is Ionization Enthalpy?
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Today, we'll explore ionization enthalpy. Does anyone know what it is?

Is it the energy needed to remove an electron from an atom?

Exactly, great job! It's the energy required to remove an electron from a gaseous atom in its ground state. We can represent this with a chemical equation: X(g) → X⁺(g) + e⁻. Here, X is our atom.

Why do we need to know about this energy?

Knowing the ionization enthalpy helps us understand how reactive an element is! A lower ionization energy means an element loses its electrons more easily, indicating higher reactivity.

Are there any factors affecting this enthalpy?

Yes, effective nuclear charge and electron shielding are key factors. As we go across a period, the nuclear charge increases, pulling electrons closer.

So, does that mean ionization increases across a period?

Exactly! The trend shows it generally increases across a period and decreases down a group. Good understanding!
Trends and Anomalies
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Let’s discuss trends in more detail. As we go left to right in a period, ionization enthalpy generally increases. Can anyone explain why?

It's because of the increasing positive charge in the nucleus?

Exactly right! This greater attraction means it takes more energy to remove an electron. But, what's the trend when going down a group?

The ionization energy decreases down a group because electrons are farther from the nucleus.

Correct! Now, can you think of elements where this trend doesn't hold?

Beryllium and boron have a weird trend. Boron's ionization is lower even though it has more protons.

Good observation! This happens due to electron-electron repulsions in the p-orbital of boron. Can anyone tell me a similar example?

Like how oxygen has lower ionization than nitrogen?

Yes! Nicely done. The repulsion between paired electrons in oxygen lowers the energy needed to remove one.
Applications and Importance
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Now, let’s discuss why understanding ionization enthalpy is important. Can anyone give me a real-world application?

It could relate to why sodium explodes in water!

Absolutely! Sodium's low ionization enthalpy means it readily loses its outermost electron, making it highly reactive. Can anyone think of another example?

Maybe how chlorine reacts so vigorously with metals?

Exactly! Chlorine has a high need for electrons and thus a high electronegativity due to its position in ionization trends. This means it tends to gain an electron easily, forming anions.

So, it’s like predicting how elements will behave in reactions?

Precisely! Understanding ionization enthalpy is crucial in predicting chemical behavior and reactivity patterns, especially in the alkali and halogen families.

What do we take away from today’s lessons about trends and reactions?

We learned that ionization enthalpy helps us predict how an element will interact in chemical reactions, influenced greatly by its atomic structure.
Introduction & Overview
Read summaries of the section's main ideas at different levels of detail.
Quick Overview
Standard
This section discusses ionization enthalpy, detailing how it measures the tendency of an atom to lose electrons. It explores trends in ionization enthalpy, including the observed general increase across a period and decrease down a group, with important implications for the reactivity and chemical properties of elements.
Detailed
Ionization Enthalpy
Ionization enthalpy (
ΔiH
) is the energy required to remove an electron from an isolated gaseous atom in its ground state. For example, the reaction can be represented as:
X(g) → X⁺(g) + e⁻.
This enthalpy change is expressed in kJ/mol and indicates how readily an atom can lose an electron, which is crucial for understanding its reactivity. The section covers several key points about ionization enthalpy:
- Trends in Ionization Enthalpy: Ionization enthalpy generally increases as we move across a period (from left to right) in the periodic table due to increasing nuclear charge, which causes a stronger attraction between the nucleus and the outer electrons. Conversely, it decreases down a group because the distance between the nucleus and the outermost electrons increases, leading to greater shielding by inner shell electrons.
- Factors Influencing Ionization Enthalpy: Key factors impacting ionization enthalpy are the effective nuclear charge and electron shielding. Effective nuclear charge increases across a period while remaining relatively constant down a group, leading to varying ionization energies.
- Exceptions in Trends: Some trends in ionization enthalpy display anomalies due to electron-electron repulsion and subshell configuration. For instance, the first ionization enthalpy of boron is lower than that of beryllium due to the different types of electrons being removed (p-electron versus s-electron). Similarly, oxygen exhibits a lower first ionization enthalpy compared to nitrogen because of increased electron repulsion among paired electrons.
Understanding these trends is essential for predicting an element's reactivity and is foundational for further studies in atomic structure and chemical bonding.
Youtube Videos




Audio Book
Dive deep into the subject with an immersive audiobook experience.
Definition of Ionization Enthalpy
Chapter 1 of 4
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
A quantitative measure of the tendency of an element to lose electron is given by its ionization Enthalpy. It represents the energy required to remove an electron from an isolated gaseous atom (X) in its ground state.
Detailed Explanation
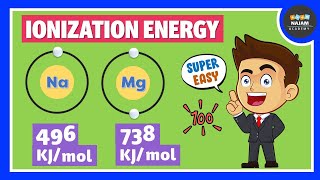
Ionization enthalpy is the energy needed to remove an electron from an atom in its gaseous state. This value helps scientists understand how easily an atom can lose an electron, which is crucial for predicting how an element will behave chemically. For example, removing an electron from sodium (Na) to form Na+ requires energy, making the ionization enthalpy a positive value.
Examples & Analogies
Think of ionization enthalpy like trying to take a ball away from a child. The child (the atom) will resist you until you provide enough effort or energy to actually take the ball (the electron) away.
First and Second Ionization Enthalpies
Chapter 2 of 4
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
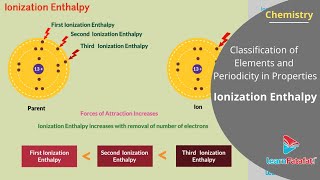
The first ionization enthalpy for an element X is the enthalpy change (∆i H) for the reaction depicted in equation 3.1.
X(g) → X+(g) + e– (3.1)
The second ionization enthalpy is the energy required to remove the second most loosely bound electron; it is the energy required to carry out the reaction shown in equation 3.2.
X+(g) → X2+(g) + e– (3.2)
Detailed Explanation
The first ionization enthalpy refers to the energy required to remove the first electron, forming a positively charged ion. The second ionization enthalpy is the energy needed to remove another electron from this ion, which is always higher due to the stronger attraction of the remaining electrons to the nucleus. In simple terms, once an electron is removed, the remaining electrons feel a stronger pull from the nucleus, making it harder to remove another one.
Examples & Analogies
Imagine trying to pull off two stickers stuck firmly to a wall. The first sticker comes off fairly easily, but once it's gone, the next sticker sticks even more strongly because there’s less surface for the remaining stickers to cling to. This is similar to how it becomes increasingly difficult to remove electrons once one has already been removed.
Trends in Ionization Enthalpy
Chapter 3 of 4
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Energy is always required to remove electrons from an atom and hence ionization enthalpies are always positive. The first ionization enthalpy will be higher than the first because it is more difficult to remove an electron from a positively charged ion than from a neutral atom.
Detailed Explanation
Ionization enthalpy generally increases across a period from left to right and decreases as we go down a group of the periodic table. This is because as you move to the right, elements have more protons in their nucleus, creating a stronger positive charge that pulls electrons closer, making them harder to remove. On the other hand, as you go down a group, the increase in electron shells keeps outer electrons farther from the nucleus, thereby reducing the ionization energy required to remove them.
Examples & Analogies
Consider a relationship analogy: a parent (the nucleus) has a stronger bond with their older child (the electron) compared to their younger child due to maturity (higher atomic number) when both are present. However, if the family moves to a larger house (going down a group), the older child's friends (additional shells) can play too and they feel less constrained, making it easier for them to leave the house (be removed).
Factors Affecting Ionization Enthalpy
Chapter 4 of 4
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
To understand these trends, we have to consider two factors: (i) the attraction of electrons towards the nucleus, and (ii) the repulsion of electrons from each other.
Detailed Explanation
The two main factors affecting ionization enthalpy include the attractive force between the nucleus and the electrons, and the repulsive forces among electrons. When an atom has more protons (greater nuclear charge), the attraction for outer electrons increases, raising the ionization enthalpy. Conversely, as repulsive forces increase due to an added electron, it becomes easier to remove an electron, thus decreasing the ionization energy needed.
Examples & Analogies
Think about a crowded room where people are standing close together (electrons). The more people there are (more electrons), the more they push against each other making it easier for one to leave the room (be removed). But as more support comes into the room (more protons), the bonds of friendship (attraction) become stronger, making it difficult for any one individual to leave.
Key Concepts
-
Ionization Enthalpy: A crucial property indicating how easily an atom can lose an electron.
-
Trends in Ionization: Increases across a period and decreases down a group, influenced by effective nuclear charge and shielding.
-
Electron-electron Repulsion: A key factor that can lead to anomalies in the expected trend of ionization enthalpies.
Examples & Applications
The ionization enthalpy of sodium is low, allowing it to react vigorously with water.
The ionization enthalpy of chlorine is high, leading to its tendency to gain electrons and form anions.
Memory Aids
Interactive tools to help you remember key concepts
Rhymes
Ionization's energy climbs, in a period defines the times.
Stories
Imagine a hero, ionizing an element, but the closer they are to the nucleus, the stronger their bond, making them harder to remove.
Memory Tools
ICE - Ionization energy Increases across a period.
Acronyms
SCHED - Shielding, Charge, Height distance (nuclear charge), Electron repulsion, Down the group to remember the factors affecting ionization.
Flash Cards
Glossary
- Ionization Enthalpy
The energy required to remove an electron from a gaseous atom in its ground state.
- Effective Nuclear Charge
The net positive charge experienced by an electron in a multi-electron atom.
- Shielding
The phenomenon where inner shell electrons reduce the effective nuclear charge felt by outer shell electrons.
- Electronelectron Repulsion
The repulsive force between electrons that affects their arrangement in orbitals.
Reference links
Supplementary resources to enhance your learning experience.
