Atomic Radius
Enroll to start learning
You’ve not yet enrolled in this course. Please enroll for free to listen to audio lessons, classroom podcasts and take practice test.
Interactive Audio Lesson
Listen to a student-teacher conversation explaining the topic in a relatable way.
Understanding Atomic Radius
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Today, we’re going to dive into the atomic radius. Can anyone tell me why understanding atomic size is important in chemistry?

It affects properties like how atoms bond with each other, right?

Exactly! The atomic radius plays a crucial role in determining the behavior of elements during chemical reactions.

How do we actually measure atomic radius since atoms are super tiny?

Great question! We estimate atomic size by looking at bond lengths between atoms. For example, in Cl2, the bond distance measures 198 pm, and we get the atomic radius of chlorine by halving that distance. Remember, this is called the 'covalent radius.'

What about metals?

Good! For metals, we talk about 'metallic radius,' which is also determined by the distance between adjacent metal atoms in the crystal.

Does the atomic radius change as we move across the periodic table?

Yes, it does! Can anyone tell me what trend we observe across a period?

The atomic radius decreases from left to right due to the increased nuclear charge!

Correct! The increased pull from the positively charged nucleus draws electrons closer, thus decreasing size.

And what happens when we move down a group?

Excellent! As we go down, the atomic radius increases due to additional energy levels. Think of it like moving down a staircase—the further you go, the higher you are from the ground.

Before we finish, let's recap: The atomic radius affects bonding, decreases across a period, and increases down a group.
Impact of Atomic Radius
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Now, let's explore how atomic radius impacts ionization energy and electronegativity. Why do you think these properties are related?

I think bigger atoms might be less attracted to their electrons, so it would be easier to remove one.

That's right! Larger atomic radii mean that the outermost electrons are further from the nucleus, resulting in lower ionization energy. Can anyone summarize the trends in ionization energy across a period?

Ionization energy increases across a period because the atomic radius decreases, making it harder to remove an electron.

Exactly! Moving on, what about electronegativity; how does atomic radius influence that?

Electronegativity increases across a period and decreases down a group!

Spot on! Atoms that are smaller can attract electrons more effectively, enhancing their electronegativity. Can anyone think of an example?

Fluorine is a really small atom and is highly electronegative.

Exactly! Smaller radius, higher electronegativity! Let's summarize: Atomic radius influences ionization energy by increasing trends from left to right, and it plays a crucial role in electronegativity as well.
Introduction & Overview
Read summaries of the section's main ideas at different levels of detail.
Quick Overview
Standard
The atomic radius has a significant influence on chemical properties, showing periodic trends based on both atomic number and position within groups of the periodic table. Understanding how atomic radius varies can enhance knowledge of other chemical properties such as ionization energy and electronegativity.
Detailed
Atomic Radius
The atomic radius is defined as a measure of the size of an atom, usually represented in picometers (pm) or angstroms (Å). Estimating the atomic radius, however, poses complications due to the lack of a solid boundary around electrons. Two common methods for measuring atomic size are
- Covalent radius: derived from the distance between two atoms bonded together, with each radius being half the bond distance.
- Metallic radius: obtained from the distance between metallic core atoms in a metallic crystal structure, again halved.
Trends in atomic radius can be observed across periods and down groups in the periodic table.
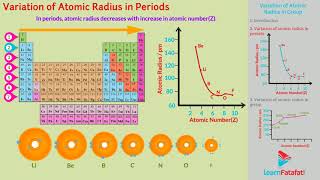
1. Across a Period: The atomic radius generally decreases because, as you move from left to right across a period, electrons are added to the same energy level while the nuclear charge (number of protons) increases. This increased nuclear charge pulls the electrons closer to the nucleus, resulting in a smaller atomic radius.
2. Down a Group: The atomic radius increases as you go down a group due to the addition of principal energy levels (n), which means valence electrons are further from the nucleus and the inner electrons shield the outer electrons from the nuclear charge.
Key observations and examples are illustrated by comparing atomic radii between groups and periods, such as lithium and sodium, or chlorine and bromine. Understanding atomic radius is foundational to grasping other related properties like ionic radius, ionization enthalpy, and electronegativity, and is crucial for interpreting chemical behavior.
Youtube Videos









Audio Book
Dive deep into the subject with an immersive audiobook experience.
Understanding Atomic Size
Chapter 1 of 5
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
You can very well imagine that finding the size of an atom is a lot more complicated than measuring the radius of a ball. Firstly, because the size of an atom (~ 1.2 Å i.e., 1.2 × 10–10 m in radius) is very small. Secondly, since the electron cloud surrounding the atom does not have a sharp boundary, the determination of the atomic size cannot be precise. In other words, there is no practical way by which the size of an individual atom can be measured.
Detailed Explanation
The atomic radius is not easily quantified because atoms are extremely small—so small that typical measuring techniques used for larger objects don't apply. We also cannot pinpoint where an atom ends, as the electrons surrounding it exist in a cloud rather than a fixed shell. This means that measuring these dimensions is inherently uncertain.
Examples & Analogies
Imagine trying to measure the size of a cloud with a ruler. Just as clouds change shape and don't have defined edges, atoms have electron clouds that make measuring their 'size' challenging.
Methods of Estimating Atomic Radius
Chapter 2 of 5
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
However, an estimate of the atomic size can be made by knowing the distance between the atoms in the combined state. One practical approach to estimate the size of an atom of a non-metallic element is to measure the distance between two atoms when they are bound together by a single bond in a covalent molecule and from this value, the 'Covalent radius' of the element can be calculated.
Detailed Explanation
To overcome the difficulty in measuring atomic size directly, scientists look at how atoms interact in bonds. In a molecule, for example, the distance between two bonded atoms represents a practical way to estimate the size of a single atom when you divide that distance by two. This is known as the covalent radius.
Examples & Analogies
Think of how we can estimate the width of a door by measuring the distance between the door frame. Similarly, we estimate atomic size by measuring the bond lengths in molecules.
Definition of Atomic Radius Types
Chapter 3 of 5
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
For example, the bond distance in the chlorine molecule (Cl2) is 198 pm and half this distance (99 pm) is taken as the atomic radius of chlorine. For metals, we define the term 'metallic radius' which is taken as half the internuclear distance separating the metal cores in the metallic crystal.
Detailed Explanation
Different atomic radii types exist: the covalent radius for non-metals and metallic radius for metals. For chlorine, its atomic radius is half of the distance between the two bonded chlorine atoms. Similarly, the distance between adjacent metal atoms in a crystal gives us the metallic radius.
Examples & Analogies
Imagine measuring a rope by folding it: the length of one fold gives us information about the whole rope. In chemistries, we measure distances in bonds to evaluate atomic sizes by halving those distances.
Trends in Atomic Radii
Chapter 4 of 5
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Two trends are obvious. We can explain these trends in terms of nuclear charge and energy level. The atomic size generally decreases across a period as illustrated for the elements of the second period. It is because within the period, the outer electrons are in the same valence shell and the effective nuclear charge increases as the atomic number increases resulting in the increased attraction of electrons to the nucleus.
Detailed Explanation
As you move left to right across the periodic table, each element has more protons (increased nuclear charge) without a corresponding increase in the distance of valence electrons from the nucleus. This leads to a stronger attraction between the nucleus and the electrons, causing the atomic radius to decrease.
Examples & Analogies
Think of it like a magnet: as you approach the fridge with a magnet, the attractive force between them increases, pulling the magnet closer. In atoms, as protons increase, they pull the surrounding electrons in tighter, shrinking atomic size.
Atomic Radius in Group Trends
Chapter 5 of 5
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Within a family or vertical column of the periodic table, the atomic radius increases regularly with atomic number. For alkali metals and halogens, as we descend the groups, the principal quantum number (n) increases and the valence electrons are farther from the nucleus. This happens because the inner energy levels are filled with electrons, which serve to shield the outer electrons from the pull of the nucleus.
Detailed Explanation
In a vertical group, as you go down the table, the atoms have more energy levels, which means the outermost electrons are farther from the nucleus. This increased distance, coupled with electron shielding from inner electrons, allows the atomic radius to increase.
Examples & Analogies
Consider how a taller person has arms that are further away from their body than a shorter person. In a similar way, as atoms get more energy levels (or layers of electrons), their 'outer arms'—the outermost electrons—get farther away, increasing atomic size.
Key Concepts
-
Atomic Radius: Measures the size of an atom; varies across periods and groups.
-
Covalent Radius: Half of the distance between two bonded atoms in a covalent bond.
-
Metallic Radius: Half of the distance between adjacent metallic atoms in a crystalline structure.
-
Ionization Energy: Energy required to remove an electron; affected by atomic radius.
-
Electronegativity: Ability of an atom to attract electrons; also influenced by atomic radius.
Examples & Applications
The atomic radius decreases from lithium to fluorine as we move across the second period.
The atomic radius increases from lithium to cesium down group 1.
Memory Aids
Interactive tools to help you remember key concepts
Rhymes
The atomic size goes down with might, as we move across the periodic light.
Stories
Once there was a tiny atom named Lithium who noticed that as he made friends with the other elements, they got smaller the further across they went, all due to the pulling power of the nucleus.
Memory Tools
Acronym ‘ARISE’ - Atomic Radius Is Smaller Everywhere (to remember trends of atomic radius).
Acronyms
PIG - Position In Groups; increases down a group.
Flash Cards
Glossary
- Atomic Radius
A measure of the size of an atom, typically represented in picometers (pm) or angstroms (Å).
- Covalent Radius
The radius determined by half the distance between two atoms bonded in a covalent bond.
- Metallic Radius
Half the distance between adjacent atoms in a metallic crystal.
- Ionization Energy
The energy required to remove an electron from an isolated gaseous atom.
- Electronegativity
The ability of an atom to attract shared electrons in a chemical bond.
Reference links
Supplementary resources to enhance your learning experience.
