Exercises
Enroll to start learning
You’ve not yet enrolled in this course. Please enroll for free to listen to audio lessons, classroom podcasts and take practice test.
Interactive Audio Lesson
Listen to a student-teacher conversation explaining the topic in a relatable way.
The Importance of the Periodic Table
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Today, we'll discuss the significance of the Periodic Table in chemistry. Can anyone tell me why the Periodic Table is so vital?

It organizes the elements based on their properties.

Exactly! It provides a systematic framework for understanding how elements relate to one another. This organization helps in predicting chemical behavior.

How does it help predict behavior?

Good question! By analyzing trends across groups and periods, scientists can foresee how different elements will engage in chemical reactions. For example, elements in the same group often have similar reactivity.

Can you give an example of a trend?

Sure! As we move down a group, metallic character increases. This means the elements become more willing to lose electrons and form cations. Remember the acronym 'ME' for Metallic Character Increasing down.

So, the table helps us in many ways?

Absolutely! The Periodic Table is not just a list; it’s a roadmap of chemical behavior. In short, it helps rationalize known chemical facts and also predicts new ones.
Mendeleev and the Development of the Periodic Table
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Let’s now talk about Dmitri Mendeleev. What do you know about his contributions?

He was the one who created the first Periodic Table, right?

Correct! Mendeleev organized elements by atomic weight and noted that properties recurring at intervals he called periodicity.

Did he leave gaps in his table?

Yes! Remarkably, he predicted the existence of elements that had not yet been discovered. For instance, he left spaces for Gallium and Germanium. Remember the term 'Predicted Elements' for his remarkable foresight.

What was the basis of his classification?

Mendeleev classified elements based on their atomic weights, but he prioritized properties. This inconsistency was corrected later with atomic numbers. Just remember: 'Weight for Order, Properties for Priority.'
Understanding Periodicity and Trends
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Can anyone describe periodic trends we observe in the Periodic Table?

Isn't it that atomic radius increases down a group?

Exactly! As you go down, you're adding energy levels, increasing the size. Remember 'AR: Always Rising down.' Can someone mention what happens as you go across a period?

The atomic radius decreases across a period?

Yes! The increased nuclear charge pulls electrons closer, reducing size. Helpful memory: 'Smaller Group, Bigger Period.' Why do we care about these trends?

It helps us predict how elements might behave in reactions!

Spot on! Understanding these trends is crucial in predicting reactions and element behavior.
Classification of Elements
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Let's move to the classification of elements into different blocks. Who can categorize the s, p, d, and f-blocks?

The s-block includes Groups 1 and 2, right?

Correct! And the p-block comprises the Group 13 to 18 elements. 'S and P: Simple Configuration.' What about d and f-block?

D-block is for transition metals and f-block is for lanthanides and actinides.

Exactly! d-block is where we fill d orbitals, and f-block includes the filling of f orbitals. Great job remembering those configurations.

What importance does this have in real-life applications?

Understanding these classifications is key for predicting chemical properties, especially for reactions and compound formation.
Introduction & Overview
Read summaries of the section's main ideas at different levels of detail.
Quick Overview
Standard
The section outlines the historical development of the Periodic Table, explains Mendeleev's contributions, and emphasizes the importance of periodicity in understanding the properties of elements. It further details the classification of elements into different block categories based on electronic configurations.
Detailed
Exercises
This section elaborates on the foundational concept of the Periodic Table, regarded as the cornerstone of chemical understanding. The Periodic Table not only organizes chemical elements but also reveals their properties and trends within groups and periods. It illustrates how elements can be classified based on their electron configurations, atomic numbers, and properties. Mendeleev's initial explorations into periodicity laid the groundwork, leading to the modern classification of elements by atomic number, which reflects their electronic structure and properties, including ionization enthalpy and atomic radii. Understanding such trends is crucial for anyone pursuing chemistry as it helps predict elemental behavior in reactions.
Youtube Videos





Audio Book
Dive deep into the subject with an immersive audiobook experience.
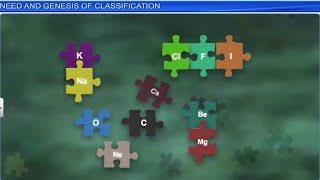
Basic Theme of Organisation
Chapter 1 of 9
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
What is the basic theme of organisation in the periodic table?
Detailed Explanation
The periodic table is organized based on the atomic number of elements. Elements are arranged in order of increasing atomic number, which reflects the number of protons in an atom’s nucleus. This arrangement shows that elements with similar properties recur at regular intervals, allowing chemists to predict the characteristics of elements based on their position in the table.
Examples & Analogies
Think of the periodic table like a library cataloging books. Just as books are arranged by author or title, making it easier to find works by the same author, elements are categorized based on their atomic structure and properties, making it easier to find and understand chemical behavior.
Mendeleev's Classification
Chapter 2 of 9
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Which important property did Mendeleev use to classify the elements in his periodic table and did he stick to that?
Detailed Explanation
Mendeleev used the atomic mass of elements as the primary property for classifying them in his periodic table. He initially arranged the elements by increasing atomic mass and grouped them based on similar chemical properties. However, he later encountered issues where some elements did not fit this arrangement correctly. For example, he placed iodine (with a lower atomic mass than tellurium) in a group based on its chemical properties rather than strictly adhering to increasing atomic mass.
Examples & Analogies
Imagine organizing a group of friends by their ages. At first, it may seem logical to line them up from youngest to oldest. But if one of your friends behaves more like someone significantly older than they are, you might decide to place them with that older group instead. Mendeleev faced a similar challenge with his classification system.
Difference Between Mendeleev's and Modern Periodic Law
Chapter 3 of 9
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
What is the basic difference in approach between the Mendeleev’s Periodic Law and the Modern Periodic Law?
Detailed Explanation
The basic difference lies in how elements are classified. Mendeleev's Periodic Law was based on atomic mass, stating that the properties of elements are a periodic function of their atomic weights. In contrast, the Modern Periodic Law states that the properties of elements are a periodic function of their atomic numbers, reflecting the number of protons rather than the total mass of the atoms. This change was validated by research in atomic structure, such as Moseley's work identifying atomic numbers.
Examples & Analogies
Consider how you might arrange a set of colored blocks. Mendeleev's arrangement would be like organizing by weight, where heavier blocks come first. In comparison, the modern approach would be like organizing the blocks in order of height, which provides a clearer understanding of their relations based on a specific physical property.
Quantum Numbers and the Sixth Period
Chapter 4 of 9
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
On the basis of quantum numbers, justify that the sixth period of the periodic table should have 32 elements.
Detailed Explanation
The sixth period contains elements with principal quantum numbers n=6. Each principal quantum shell can hold a maximum number of electrons described by the formula 2n². For n=6, this means a maximum of 72 electrons theoretically can fit. However, due to the filling of the f-block elements (the lanthanides), the actual number of elements that appear is 32, as the filling order of sublevels modifies how space is allocated.
Examples & Analogies
Think of a concert hall that can hold a specific number of seats based on sections (like_groups of elements in the periodic table). Each section (principal quantum level) can hold a certain number of people (electrons). While the concert hall might have an ideal maximum capacity, the setup and design might lead to certain areas filling up with specific arrangement patterns, resulting in the actual attendance being lower than that of the total capacity.
Locating Elements by Atomic Number
Chapter 5 of 9
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
In terms of period and group where would you locate the element with Z =114?
Detailed Explanation
Element Z = 114, known as Flerovium, is located in the seventh period and fourteenth group of the periodic table. Elements in the same group share similar chemical properties, reflective of their electron configurations. As such, Flerovium would exhibit properties characteristic of other group 14 elements, similar to those of lead and tin.
Examples & Analogies
Locating an element on the periodic table is similar to mapping out locations on a map based on coordinates (like a grid). Just as the coordinates define a specific spot on the map, the atomic number helps pinpoint where the element is laid out on the table.
Atomic Number of Elements
Chapter 6 of 9
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Write the atomic number of the element present in the third period and seventeenth group of the periodic table.
Detailed Explanation
The element located in the third period and seventeenth group of the periodic table is Chlorine, which has an atomic number of 17. This position indicates that it is in the third row and is part of the halogen group known for its reactivity and electronegativity.
Examples & Analogies
Asking for the atomic number is like inquiring about the jersey number of a player on a sports team. Each player (element) has a unique identifier (atomic number) that distinguishes them in the roster (periodic table), representing their specific characteristics.
Naming Elements
Chapter 7 of 9
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Which element do you think would have been named by (i) Lawrence Berkeley Laboratory (ii) Seaborg’s group?
Detailed Explanation
Based on historical precedence, elements in the vicinity of the Lawrence Berkeley Laboratory include elements such as Californium, which was discovered there. Similarly, Glenn T. Seaborg played a significant role in the discovery of several transuranium elements, and element 106 is named Seaborgium in his honor.
Examples & Analogies
Think of naming rights in sports arenas, where a venue might be named after a significant contributor or donor. The practice emphasizes the impact the influential person's contribution (like discovery in chemistry) to the facility and its legacy.
Similar Properties within Groups
Chapter 8 of 9
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Why do elements in the same group have similar physical and chemical properties?
Detailed Explanation
Elements in the same group share similar physical and chemical properties due to their similar electron configuration, particularly in their outermost shells. This similarity results in comparable reactivities and predictions of behavior in chemical reactions. For example, alkali metals all have one electron in their outer shell, making them highly reactive and similar in behavior despite their differences in atomic mass.
Examples & Analogies
Think of a family where siblings share similar traits (like eye colors or personalities). In chemistry, groups within the periodic table are like familial traits, where members exhibit shared characteristics and behaviors due to their 'genes' (electron configurations).
Understanding Atomic and Ionic Radius
Chapter 9 of 9
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
What do you understand by atomic radius and ionic radius?
Detailed Explanation
Atomic radius refers to the size of an atom, typically measured from the nucleus to the boundary of the surrounding cloud of electrons. Ionic radius, however, pertains to the size of an atom once it has gained or lost electrons and formed an ion. Cations (positively charged ions) have smaller radii due to fewer electrons, while anions (negatively charged ions) are larger due to increased electron-electron repulsion.
Examples & Analogies
Imagine a balloon (the atom) that gets smaller when you let some air out (ionization) versus when you blow more air into it (an ion being formed). That illustrates chemically how elements behave and
Key Concepts
-
Periodic Table: An organized arrangement of elements based on atomic number and properties.
-
Atomic Number: The number of protons in an atom's nucleus; fundamental to identifying elements.
-
Trends: Observable patterns in properties like atomic radius and ionization energy.
-
Mendeleev's Contributions: Early classifications and predictions of undiscovered elements.
Examples & Applications
Mendeleev left gaps for missing elements in his periodic table, predicting the properties of gallium and germanium, which were later found.
As we descend group 1 (alkali metals), we observe an increase in metallic character, from lithium to cesium.
Memory Aids
Interactive tools to help you remember key concepts
Rhymes
Elements arranged with logic so fine, predict their behavior across the line.
Stories
In the kingdom of Chemistry, Mendeleev once dreamed of a table where all elements could belong, understanding that order would reveal their true nature.
Memory Tools
Remember 'A-T-M' for Atomic Number, Trends, and Mendeleev, key elements of the Periodic Table.
Acronyms
P.O.R.T
Periodic Table
Organization
Reactivity
Trends.
Flash Cards
Glossary
- Periodic Table
A tabular arrangement of chemical elements based on their atomic number, electron configurations, and recurring chemical properties.
- Atomic Number
The number of protons in the nucleus of an atom, which determines the element's identity.
- Metallic Character
The tendency of an element to lose electrons, which increases as you move down a group in the Periodic Table.
- Periodic Trends
Patterns observed in elemental properties (such as atomic radius, ionization energy) that change across periods and groups in the Periodic Table.
- Valence Electrons
Electrons that are in the outermost shell of an atom and are involved in chemical bonding.
Reference links
Supplementary resources to enhance your learning experience.
