Conductance of Electrolytic Solutions
Enroll to start learning
You’ve not yet enrolled in this course. Please enroll for free to listen to audio lessons, classroom podcasts and take practice test.
Interactive Audio Lesson
Listen to a student-teacher conversation explaining the topic in a relatable way.
Introduction to Conductance and Resistivity
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Today we'll discuss conductance and resistivity. To start, can anyone tell me how we define resistivity?

Resistivity is a measure of how much a material resists the flow of electric current, right?

"Exactly! Resistivity (
Measuring Conductivity
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Now, let's discuss how we measure the conductivity of solutions. Who can tell me the equipment we might use?

We typically use a conductivity meter or a specially designed conductivity cell.

Exactly! This device consists of electrodes that measure the flow of electric current through the solution. What do you think is a challenge in measuring conductivity?

Passing direct current could change the composition of the solution.

Very true! That's why we often use alternating current for these measurements. What affects conductivity in solutions?

Factors like ion concentration, size, and the temperature of the solution.

Great summary! Remember, conductivity generally increases with increased ion concentration.
Kohlrausch's Law
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Next, let's delve into Kohlrausch's law. Can someone explain what this law states?

It states that the molar conductivity of an electrolyte at infinite dilution is the sum of the contributions from its ions.

Exactly! So, if we know the contributions of the individual ions, we can predict the molar conductivity of compounds. Can anyone provide an example?

For sodium chloride, we add the contributions of Na+ and Cl– to get the total!

Correct! It's like building with blocks! Each ion adds its own strength to the overall structure. Remembering that should help you with calculations.
Applications and Importance
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Finally, let's talk about how these concepts are applied! Why are conductivity and resistivity important in electrochemistry?

They help us understand how batteries and electrochemical cells function.

Exactly! High conductivity can imply a better performance in cells. How about in environmental contexts?

It helps assess water quality and the presence of contaminants!

Spot on! Conductivity assessments are essential for monitoring ecological health. Each of you is gaining a solid grasp here!
Recap and Reinforcement
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Let's summarize what we learned. What are the key relationships between resistivity and conductivity?

Resistivity is the opposition to current, while conductivity shows how well current can flow.

Kohlrausch's law sums individual ions for molar conductivity!

Excellent! Keep these points in mind as we move forward. Real-world applications are where this knowledge becomes powerful.
Introduction & Overview
Read summaries of the section's main ideas at different levels of detail.
Quick Overview
Standard
In this section, we explore the definitions of resistivity, conductivity, and molar conductivity, and their significance in electrochemical cells. We examine the principles of electrolysis, the impact of concentration changes on conductivity, and the practical methods used to measure these properties in solutions.
Detailed
Detailed Summary
This section delves into the concepts of conductance and resistivity, essential components in understanding electrochemical processes. Conductivity (
k
greek) represents the ability of a material to conduct electric current, while resistivity (
r
greek) indicates how strongly a material opposes the flow of electricity.
Flush with practical applications, these concepts are pivotal in the functioning of electrochemical cells.
Key aspects such as the relationship between resistance, length, and cross-sectional area of conductors are also covered. The section elaborates on Faraday's laws of electrolysis, outlining how different metals can either gain or lose electrons based on their electrode potential, which is crucial for understanding galvanic and electrolytic cells.
In addition, the variation of conductivity (described by Kohlrausch's law) and molar conductivity with electrolyte concentration highlights the significance of these ratios in real-world applications, such as battery technology and fuel cells. The section concludes by emphasizing the considerations in measuring these properties accurately within laboratory settings.
Youtube Videos









Audio Book
Dive deep into the subject with an immersive audiobook experience.
Definition of Electrical Resistance and Resistivity
Chapter 1 of 4
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
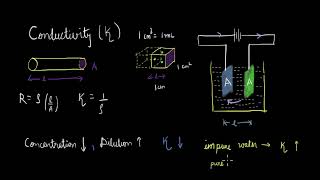
The electrical resistance is represented by the symbol ‘R’ and it is measured in ohm (Ω), which in terms of SI base units is equal to (kg m²)/(s³ A²). The electrical resistance of any object is directly proportional to its length, l, and inversely proportional to its area of cross section, A. That is,
R ∝ l or R = ρ (l/A)
Detailed Explanation
Electrical resistance (R) is a measure of how much an object opposes the flow of electric current. It depends on two geometric factors: the length of the conductor and the cross-sectional area of the conductor. The longer the conductor, the greater the resistance, because electrons have to travel a longer distance, facing more collisions. In contrast, a larger cross-sectional area allows more electrons to move through simultaneously, reducing resistance. The resistivity (ρ, rho) is a material property that indicates how strongly a material resists the flow of current.
Examples & Analogies
Think of water flowing through a pipe. If the pipe is long and narrow (high resistance), water will struggle to flow through it compared to a short, wide pipe (low resistance). The same principle applies to electrical conductors.
Conductance and Conductivity
Chapter 2 of 4
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
The inverse of resistance, R, is called conductance, G, and we have the relation:
G = 1/R = k (where k is conductivity). The SI unit of conductance is siemens, represented by the symbol ‘S’ and is equal to ohm–1 (also known as mho) or W–1. The inverse of resistivity, called conductivity (specific conductance) is represented by the symbol, k (kappa). The SI units of conductivity are S m–1.
Detailed Explanation
Conductance (G) is the ability of a material to allow the flow of electric current. While resistance opposes current, conductance indicates how easily electricity can pass through a material. Conductivity (k) measures how well a specific material allows electricity to flow through it. Higher conductivity means electrons can move freely, leading to lower resistance.
Examples & Analogies
Imagine a highway. If the highway is wide and has no toll booths (high conductance), cars (electrons) can flow freely. But if it's a narrow, congested road with toll booths (high resistance), cars will move slowly.
Measurement of Conductivity
Chapter 3 of 4
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
However, for measuring the resistance of an ionic solution, we face challenges; first, DC changes the composition of the solution. The first difficulty is resolved by using an alternating current (AC) source of power. The second problem is solved by using a specially designed vessel called conductivity cell.
Detailed Explanation
Measuring the conductivity of ionic solutions can be tricky because applying direct current can alter the chemical makeup of the solution. To bypass this issue, alternating current (AC) is used, which doesn't change the solution's composition. A conductivity cell is a device specifically designed to measure how well a solution conducts electricity, using these principles.
Examples & Analogies
Think of measuring the flow of a river. Using DC is like trying to measure the flow while constantly throwing rocks in the river (which changes it). Using AC is like measuring while ensuring the rocks are taken out after measuring, preventing any change.
Factors Affecting Conductivity
Chapter 4 of 4
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
The conductivity of electrolytic (ionic) solutions depends on:
(i) The nature of the electrolyte added
(ii) Size of the ions produced and their solvation
(iii) The nature of the solvent and its viscosity
(iv) Concentration of the electrolyte
(v) Temperature (it increases with the increase of temperature).
Detailed Explanation
Several factors influence the conductivity of ionic solutions. For example, the type of electrolyte affects how many ions are produced, which is vital because more ions typically mean better conductivity. The size of the ions and how they interact with the solvent also play a role—larger ions might move slower. Additionally, as temperature increases, ion mobility improves, leading to higher conductivity.
Examples & Analogies
Think of conductivity as a water slide. A more slippery surface (like a good solvent) helps water (ions) flow faster. In contrast, if the slide is rough (like a viscous solvent), water will flow slower. More people (higher concentration) on the slide also means faster flow.
Key Concepts
-
Conductivity: A measure of a material's ability to conduct electric current.
-
Resistivity: A measure of a material's opposition to current flow.
-
Molar Conductivity: Conductivity normalized by the concentration of the solution.
-
Kohlrausch's Law: The relation of molar conductivity to ion contributions at infinite dilution.
Examples & Applications
The resistance of a copper wire is low due to high conductivity, making it suitable for electrical applications.
Kohlrausch's Law can predict the behavior of a salt solution based on its ion concentrations.
Memory Aids
Interactive tools to help you remember key concepts
Rhymes
Conductivity flows, resistivity slows, that's how charge goes!
Stories
Once there were two rivers, one fast and one slow. The fast river (conductivity) powered a village, while the slow (resistivity) held back boats.
Memory Tools
C.R.M. stands for Conductivity, Resistivity, Molar conductivity.
Acronyms
C for Conductivity, R for Resistivity, M for Molar conductivity.
Flash Cards
Glossary
- Conductivity
The ability of a material to conduct electric current, typically measured in siemens.
- Resistivity
The inherent property of a material to resist the flow of electric current, measured in ohm-meters.
- Molar Conductivity
Conductivity of a solution per unit concentration, usually expressed as S cm² mol–1.
- Kohlrausch's Law
A principle stating that molar conductivity at infinite dilution is equal to the sum of the molar conductivities of its constituent ions.
- Electrolytic Solution
A solution that conducts electricity due to the presence of ions.
Reference links
Supplementary resources to enhance your learning experience.
