Equilibrium Constant from Nernst Equation
Enroll to start learning
You’ve not yet enrolled in this course. Please enroll for free to listen to audio lessons, classroom podcasts and take practice test.
Interactive Audio Lesson
Listen to a student-teacher conversation explaining the topic in a relatable way.
Introduction to Electrochemical Cells
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Today we will start with electrochemical cells. Can anyone tell me what an electrochemical cell is?

Isn't it a device that converts chemical energy into electrical energy?

Exactly! There are two types of electrochemical cells: galvanic cells and electrolytic cells. Galvanic cells convert chemical energy from spontaneous reactions into electrical energy, while electrolytic cells use electrical energy to conduct non-spontaneous reactions. Can anyone give an example of each type?

A Daniell cell is an example of a galvanic cell!

And electrolysis of water would be an example of an electrolytic cell!

Great examples! Remember: "Galvanic = give energy, electrolytic = use energy."

To summarize, galvanic cells convert chemical energy to electrical energy from spontaneous reactions, while electrolytic cells carry out non-spontaneous reactions using electrical energy.
Nernst Equation and Cell Potential
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Now, let's dive deeper into calculating the cell potential. Does anyone remember the Nernst equation?

Yes! It’s E = E° - rac{RT}{nF} imes ln(Q). But what does each variable mean?

Excellent question! In this equation, E is the cell potential, E° is the standard cell potential, R is the universal gas constant, T is the temperature in Kelvin, n is the number of moles of electrons, F is Faraday's constant, and Q is the reaction quotient. Let's do a mini-quiz: if E° is 1.1 V, and we have conditions where T = 298 K, n = 2, and Q is 0.01, what is E?

Should I plug in the values and solve?

Exactly! And remember to use consistent units while calculating. This will help reinforce the relationships between concentration changes and cell potential.

So, when conditions change in our electrochemical cell, the cell potential will shift, reflecting those changes.
Connection Between Gibbs Free Energy and Equilibrium Constant
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Today’s focus is on Gibbs free energy. How do we relate it to our electrochemical systems?

Is it related to the spontaneity of the reaction?

Correct! The relationship is given by ΔG = -nFE. When E is positive, ΔG is negative, showing that the reaction is spontaneous. Now, who remembers how this relates to equilibrium constant K?

We also have ΔG = -RT ln(K), right? So positive E means larger K?

That’s correct! Therefore, a large E indicates a favored reaction at equilibrium. Let’s summarize this relationship: positive E means a spontaneous reaction and a larger equilibrium constant!
Practical Applications: Batteries and Corrosion
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Let's connect our understanding so far to real-world applications. Who can relate an electrochemical cell to a battery?

Batteries are like portable galvanic cells!

Exactly! They rely on reversible chemical reactions. Can anyone discuss electrolysis in the context of corrosion?

Corrosion is basically metal getting oxidized due to the electrochemical reactions in the environment.

Well said! Understanding the principles of electrochemistry helps us design better batteries and find ways to reduce corrosion. Let’s wrap up with a key point: corrosion can be minimized by preventive measures like coatings and sacrificial anodes.
Introduction & Overview
Read summaries of the section's main ideas at different levels of detail.
Quick Overview
Standard
This section outlines essential concepts in electrochemistry, such as galvanic and electrolytic cells, the Nernst equation, standard electrode potentials, and how they relate to Gibbs free energy and equilibrium constants. It further explores practical applications including battery function and corrosion.
Detailed
Detailed Summary of the Equilibrium Constant Section
Electrochemistry is rooted in the understanding of redox reactions and the energy produced through spontaneous chemical processes. Galvanic cells transform chemical energy into electrical energy, while electrolytic cells utilize electrical energy to instigate non-spontaneous reactions.
The Daniell cell exemplifies a galvanic cell where zinc and copper undergo oxidation and reduction respectively, generating a cell potential (emf) that can be computed with the Nernst equation:
E = E° - rac{RT}{nF} imes ln(Q)
At equilibrium, the concentrations of reactants and products remain constant, establishing the relationship between cell potential and the equilibrium constant (K) according to:
E° = rac{RT}{nF} imes ln(K)
Here, the equilibrium constant connects chemical thermodynamics to electrochemical systems, highlighting how standard conditions and electrode potentials guide predictions about reaction spontaneity and feasibility. The section also touches on the significance of resistivity, conductivity, and molar conductivity in chemical solutions, concluding with an overview of practical applications such as battery technology and corrosion.
Through thorough analysis of electrode potentials, students gain insight into how electrochemical principles govern numerous chemical processes, underscoring the balance between thermodynamics and electrochemistry.
Youtube Videos








Audio Book
Dive deep into the subject with an immersive audiobook experience.
Understanding Equilibrium in Reactions
Chapter 1 of 3
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
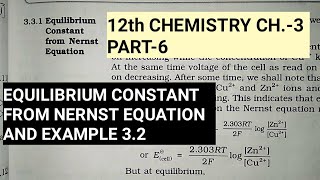
If the circuit in Daniell cell (Fig. 2.1) is closed then we note that the reaction Zn(s) + Cu2+(aq) fi Zn2+(aq) + Cu(s) takes place and as time passes, the concentration of Zn2+ keeps on increasing while the concentration of Cu2+ keeps on decreasing. At the same time, voltage of the cell as read on the voltmeter keeps on decreasing. After some time, we shall note that there is no change in the concentration of Cu2+ and Zn2+ ions and at the same time, voltmeter gives zero reading. This indicates that equilibrium has been attained.
Detailed Explanation
In electrochemical reactions, equilibrium refers to the point where the rate of the forward reaction (oxidation and reduction in the Daniell cell) equals the rate of the reverse reaction. Here, as zinc converts to zinc ions and copper ions convert to solid copper, the concentration of reactants and products changes until no further net change occurs. This state of balance means the electrochemical cell produces no voltage, indicating all reactants are in
Examples & Analogies
Think of it like balancing a seesaw. If one side goes down (more zinc ions forming), the other should go up (more solid copper forming) until they are perfectly balanced. Just like in a seesaw, when both sides are equal, the reaction reaches equilibrium.
Nernst Equation and Electrode Potential
Chapter 2 of 3
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
In this situation the Nernst equation may be written as: E = 0 = Eo – 2.303RT logZn2+ (cell) 2F [Cu2+], or Eo = log[Zn2+]/[Cu2+]. This shows the relationship between the standard cell potential and concentrations at equilibrium.
Detailed Explanation
The Nernst equation helps us understand how changes in concentrations influence the electrode potential of a cell. At equilibrium (when the reaction stops changing), the cell potential equals zero. The expression shows that if you substitute the concentrations of Zn2+ and Cu2+ ions, it helps calculate the standard potential of the Daniell cell. As concentration of reactants increases or decreases, you can predict how this affects the voltage of the cell.
Examples & Analogies
Imagine inflating a balloon. If you add air (increase concentration), it expands (increases potential) until it can't hold any more at maximum capacity (equilibrium). This is similar to how the Nernst equation predicts changes in voltage based on ion concentrations, allowing you to 'see' the potential of the cell.
Connection Between Equilibrium Constant and Cell Potential
Chapter 3 of 3
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Thus, equilibrium constants of the reaction, difficult to measure otherwise, can be calculated from the corresponding Eo value of the cell.
Detailed Explanation
This statement emphasizes how we can derive the equilibrium constant, K, of a chemical reaction from the standard electrode potential, Eo, of its electrochemical reaction. The relationship between the standard potential and the equilibrium concentration at this balance point allows us to calculate K without direct measurement, which is often challenging.
Examples & Analogies
Think of hormones and their effects in the body. Just as the concentrations at which hormones function effectively can indicate overall health and balance, in a chemical reaction, those concentrations are directly captured in the equilibrium constant derived from the electrochemical potential.
Key Concepts
-
Electrochemical Cells: Devices that convert chemical energy into electrical energy.
-
Nernst Equation: Used to calculate cell potential based on concentration and temperature.
-
Gibbs Free Energy: Indicates reaction spontaneity and is related to cell potential.
-
Equilibrium Constant (K): Defines the ratio of products to reactants at equilibrium.
-
Standard Electrode Potential: The standard measure for comparing electrode potentials.
Examples & Applications
In a Daniell cell, zinc undergoes oxidation while copper undergoes reduction, generating a specific potential.
Using the Nernst equation, we can compute the potential of a cell at various concentrations, demonstrating its variance based on reactant and product availability.
Memory Aids
Interactive tools to help you remember key concepts
Rhymes
When a cell is gal-vanic, energy it will create, but for electrolysis, power comes from a state!
Stories
Imagine a battery as a river, flowing spontaneously, while an electrolytic cell pulls up water uphill with electrical energy!
Memory Tools
Gibbs’ Energy Goes Negative (ΔG<0) as Electrode Potential (E) is Positive (E>0).
Acronyms
E = E° - RT/nF, remember it as 'Every Electrode Must Favor'!
Flash Cards
Glossary
- Electrochemical Cell
A device that converts chemical energy to electrical energy and vice versa.
- Nernst Equation
An equation that relates cell potential to the concentration of reactants and products.
- Gibbs Free Energy
A thermodynamic quantity that indicates the spontaneity of a process.
- Equilibrium Constant (K)
A numerical value that expresses the ratio of products to reactants at equilibrium.
- Cell Potential (E)
The voltage developed by an electrochemical cell.
- Standard Electrode Potential
The potential of a specific electrode measured under standard conditions.
- Electrolytic Cell
A cell that uses electrical energy to drive a non-spontaneous reaction.
- Conductivity
A measure of a material's ability to conduct electricity.
- Molar Conductivity
The conductivity of a solution divided by its molar concentration.
- Galvanic Cell
An electrochemical cell that generates electrical energy from spontaneous chemical reactions.
Reference links
Supplementary resources to enhance your learning experience.
