Corrosion
Enroll to start learning
You’ve not yet enrolled in this course. Please enroll for free to listen to audio lessons, classroom podcasts and take practice test.
Interactive Audio Lesson
Listen to a student-teacher conversation explaining the topic in a relatable way.
What is Corrosion?
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Today, we are going to explore the concept of corrosion. Can anyone explain what corrosion is?

Isn't it when metals rust or deteriorate over time?

That's right! Corrosion is essentially the deterioration of metals due to electrochemical reactions. It commonly leads to rusting, particularly in iron.

What kind of reactions are involved?

Good question! The main reaction involves oxidation of the metal. For iron, it can be represented as: 2Fe(s) → 2Fe²⁺(aq) + 4e⁻. The electrons lost must find a way to recombine, usually with oxygen or moisture.

So it forms Fe²⁺ ions and rust, right?

Exactly! The iron reacts with oxygen and water, resulting in rust, which severely weakens structures.

Can you remind us of the overall reaction?

Certainly! The overall reaction could be simplified as: 2Fe(s) + O₂(g) + 4H⁺(aq) → 2Fe²⁺(aq) + 2H₂O(l). Remember this as it encapsulates the process!
Economic Impact of Corrosion
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Now that we understand what corrosion is, let’s talk about its implications. Why might corrosion be a concern economically?

It probably costs a lot to repair or replace corroded structures, right?

Correct! Corrosion can lead to billions in damages globally every year, affecting infrastructure projects and increasing maintenance costs.

Are there specific examples of structures affected by corrosion?

Yes! Bridges, ships, pipelines, and buildings are heavily affected. Understanding corrosion is crucial for engineers to maintain these systems safely.

That's really important, especially for public safety.

Absolutely! Which brings us to prevention methods. How do you think we could prevent corrosion?
Prevention of Corrosion
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Let’s discuss how we can prevent corrosion. What methods do you all think are effective?

Using paint or coatings to cover the metal?

Yes, protective coatings are a first line of defense! They prevent moisture and air from contacting the metal surface.

I've heard of galvanizing. How does that work?

Great! Galvanizing involves coating iron with zinc, which acts sacrificially. Zinc will corrode before the iron does, protecting it. Remember the mnemonic 'Zinc Saves Iron!'

What if we added Mg or other metals?

Good thinking! Using sacrificial electrodes is another method, where a more reactive metal is used to corrode in place of the iron.

What are some common sacrificial metals?

Magnesium and zinc are common choices due to their reactivity. Always remember: preventive measures can save a lot of cost in structural integrity!
Introduction & Overview
Read summaries of the section's main ideas at different levels of detail.
Quick Overview
Standard
This section discusses the phenomenon of corrosion, focusing on how metals like iron experience oxidation through electrochemical reactions, leading to conditions like rusting. Various methods to prevent corrosion are also highlighted.
Detailed
Corrosion Overview
Corrosion is the gradual process of deterioration of metals due to reaction with environmental factors such as moisture and oxygen. This section delves into:
- Definition and Importance: Corrosion is defined as the formation of oxides or other salts on metallic surfaces leading to significant economic loss and structural failures in buildings and vehicles.
- Electrochemical Nature: The process is essentially an electrochemical phenomenon involving oxidation transformations. For instance, iron in the presence of oxygen and water oxidizes, resulting in rust (iron oxide).
- Chemical Reactions:
- Oxidation Reaction:
- 2Fe(s) → 2Fe²⁺(aq) + 4e⁻
- Reduction Reaction:
- O₂(g) + 4H⁺(aq) + 4e⁻ → 2H₂O(l)
- Overall Reaction: This results in corrosion expressed through the equation:
- 2Fe(s) + O₂(g) + 4H⁺(aq) → 2Fe²⁺(aq) + 2H₂O(l)
- Prevention Strategies: Various methods to mitigate corrosion include:
- Protective coatings, such as paints or galvanizing with zinc.
- Using sacrificial metals, where a more reactive metal (like zinc) is used to protect less reactive metals from corrosion.
Understanding corrosion is integral not only for economic reasons but also for safety in structural engineering.
Youtube Videos








Audio Book
Dive deep into the subject with an immersive audiobook experience.
Introduction to Corrosion
Chapter 1 of 4
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Corrosion slowly coats the surfaces of metallic objects with oxides or other salts of the metal. The rusting of iron, tarnishing of silver, development of green coating on copper and bronze are some examples of corrosion. It causes enormous damage to buildings, bridges, ships, and to all objects made of metals especially iron. We lose crores of rupees every year on account of corrosion.
Detailed Explanation
Corrosion is a natural process that involves the deterioration of metals as they react with their environment. It commonly manifests when metals, such as iron, come into contact with moisture and air, leading to oxidation. For instance, rusting of iron forms a layer of iron oxide (rust) which is not only unsightly but also weakens the metal, leading to structural failures in buildings and bridges.
Examples & Analogies
Imagine leaving a bicycle outside in the rain without any cover. Over time, the metal parts will start to rust, making the bike difficult to use or even unsafe. The same happens to steel structures like bridges and buildings if not properly protected.
Electrochemical Process of Corrosion
Chapter 2 of 4
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
In corrosion, a metal is oxidised by loss of electrons to oxygen and formation of oxides. Corrosion of iron (commonly known as rusting) occurs in presence of water and air. The chemistry of corrosion is quite complex but it may be considered essentially as an electrochemical phenomenon.
Detailed Explanation
Corrosion can be viewed as an electrochemical reaction where oxidation and reduction occur simultaneously. When iron rusts, it loses electrons (oxidation) and reacts with oxygen in the presence of water, resulting in the formation of rust. The overall reaction can be simplified: iron oxidizes to iron ions, while oxygen is reduced. This electrochemical process leads to the deterioration of the metal.
Examples & Analogies
Consider your car. If you ignore surface scratches and allow moisture to accumulate, those areas will oxidize and lead to rusting. The process of rusting is like a small battery forming on the metal, where certain parts of the metal lose electrons (oxidation), and other parts are accepting electrons (reduction).
Rusting Reaction
Chapter 3 of 4
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
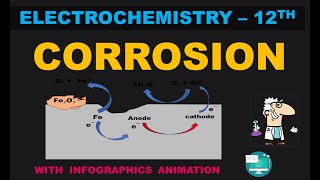
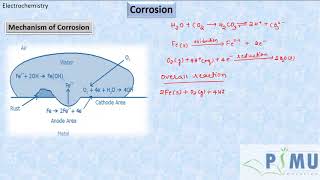
At a particular spot of an iron object, oxidation takes place and that spot behaves as an anode. The reaction is: Anode: 2 Fe (s) → 2 Fe2+ + 4 e–. Electrons released at the anodic spot move through the metal and go to another spot on the metal and reduce oxygen in the presence of H+ (which is believed to be available from H2CO3 formed due to dissolution of carbon dioxide from air into water). This spot behaves as a cathode with the reaction: Cathode: O2(g) + 4 H+(aq) + 4 e– → 2 H2O(l). The overall reaction being: 2Fe(s) + O2(g) + 4H+(aq) → 2Fe2 +(aq) + 2 H2O(l).
Detailed Explanation
When iron rusts, the oxidation process is localized; an anodic reaction occurs where iron atoms lose electrons and become iron ions. These electrons then flow to another area of the metal (the cathode), where they facilitate the reduction of oxygen in water, leading to the formation of hydroxide ions and ultimately rust. The interplay of these two reactions is what underlies corrosion.
Examples & Analogies
Think of a battery where one side starts to oxidize (anode) and another side reduces (cathode). In a car, the area where paint is chipped down to the metal acts like the anode, oxidizing, and the surrounding moisture acts as the cathode where reduction happens, leading to rust formation.
Prevention of Corrosion
Chapter 4 of 4
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Prevention of corrosion is of prime importance. It not only saves money but also helps in preventing accidents such as a bridge collapse or failure of a key component due to corrosion. One of the simplest methods of preventing corrosion is to prevent the surface of the metallic object from coming in contact with the atmosphere.
Detailed Explanation
To mitigate corrosion, many strategies can be employed, including physical barriers like paint or coatings that separate the metal from the corrosive environment. Other techniques involve galvanization where metals are plated with a more reactive metal like zinc that will corrode first, protecting the underlying iron. The key goal is to minimize the metal's exposure to moisture and oxygen, principal agents of corrosion.
Examples & Analogies
Like painting a fence to protect the wood from water and sunlight, applying a protective coating on metal surfaces helps prevent corrosion. Using a sacrificial anode in pipelines or boats is like sending a 'bodyguard' that willingly gets damaged for the sake of the main structure.
Key Concepts
-
Corrosion leads to the oxidation of metals.
-
The process is electrochemical, involving oxidation and reduction reactions.
-
Iron rusting is a commonly observed form of corrosion.
-
Prevention methods include protective coatings and sacrificial electrodes.
Examples & Applications
Rusting of iron is a common example of corrosion.
The tarnishing of silver due to oxidization is another form of corrosion.
Copper alloys developing a green patina from corrosion is a typical sight in historical buildings.
Memory Aids
Interactive tools to help you remember key concepts
Rhymes
Iron can rust and corrode, moisture and air take a toll on the road.
Stories
Once in a town, a rusty bridge made many frown. Engineers found the cure, a sacrifical metal sure!
Memory Tools
Remember 'Rust Never Sleeps' to recall how quickly corrosion can damage metals.
Acronyms
CORRODE
Chemicals Oxidize
Rust Reacts
Resulting in Damage Everywhere.
Flash Cards
Glossary
- Corrosion
The gradual deterioration of materials, particularly metals, caused by electrochemical reactions with their environment.
- Oxidation
The loss of electrons from a substance during a chemical reaction, typically resulting in an increase in oxidation state.
- Anode
The electrode at which oxidation occurs in an electrochemical cell.
- Cathode
The electrode at which reduction occurs in an electrochemical cell.
- Sacrificial Electrode
A metal electrode that is more reactive than another metal and corrodes first to protect the other metal.
- Rust
A common term for the corrosion of iron, typically resulting in a flaky, reddish-brown substance (iron oxides).
Reference links
Supplementary resources to enhance your learning experience.
