Daniell Cell and its Functioning
Enroll to start learning
You’ve not yet enrolled in this course. Please enroll for free to listen to audio lessons, classroom podcasts and take practice test.
Interactive Audio Lesson
Listen to a student-teacher conversation explaining the topic in a relatable way.
Introduction to Daniell Cell
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Today, we're going to explore the Daniell cell, a type of electrochemical cell that converts chemical energy into electrical energy. Can anyone tell me what a redox reaction is?

It's a reaction where oxidation and reduction occur simultaneously.

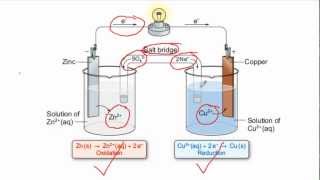
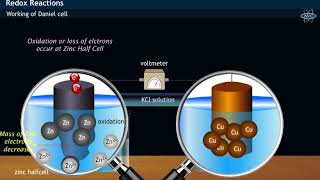
Correct! In a Daniell cell, we have the reactions: Zn oxidizing and Cu<sup>2+</sup> reducing. Can anyone describe what happens at the anode and the cathode?

At the anode, zinc loses electrons, and at the cathode, copper gains electrons.

Exactly! Remember: Anode is where oxidation occurs, which you could remember with the mnemonic AN OX. Great work!

What does the salt bridge do?

Excellent question! The salt bridge maintains the balance of charge in the half-cells by allowing ions to flow. In short, it keeps the cell neutral while the reaction takes place.
Chemical Reactions in the Daniell Cell
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Let’s look closer at the reactions in the Daniell cell: Zn(s) + Cu<sup>2+</sup>(aq) → Zn<sup>2+</sup>(aq) + Cu(s). Who can identify the oxidation and reduction half-reactions?

The oxidation half-reaction is Zn(s) → Zn<sup>2+</sup>(aq) + 2e<sup>–</sup>, and the reduction half-reaction is Cu<sup>2+</sup>(aq) + 2e<sup>–</sup> → Cu(s).

Fantastic! Remembering the half-reactions helps us understand how electrons flow. If we think of this in terms of electron flow, what do we observe in terms of voltage?

When the reaction goes spontaneously, the Daniell cell can produce voltage until concentration changes.

That's right! The standard potential of the Daniell cell is 1.1 V. You could also use the acronym E=1.1 to remember this. Any questions on this?
Applications and Importance
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Now that we've covered how the Daniell cell works, can someone explain its practical significance in daily life?

Batteries! They convert the same kind of chemical energy into electrical energy.

Exactly! These concepts are fundamental to understanding batteries that power many of our devices. Can anyone think of a common battery type made using these principles?

Lead-acid batteries!

Excellent example! Lead-acid batteries also utilize the principles of electrochemistry to produce energy. Remember, understanding these cells is crucial for future technologies.
Introduction & Overview
Read summaries of the section's main ideas at different levels of detail.
Quick Overview
Standard
Daniell cells are electrochemical devices that generate electrical energy via spontaneous redox reactions. This section discusses the construction, functioning, and the underlying chemical reactions of Daniell cells, along with their significance in electrochemistry.
Detailed
Detailed Summary of Daniell Cells
Daniell cells are a form of galvanic cell which convert the chemical energy released from a spontaneous redox reaction into electrical energy. This section outlines the operation of the Daniell cell, starting with the fundamental redox reaction:
- Zn(s) + Cu2+(aq) → Zn2+(aq) + Cu(s)
In a Daniell cell, the anode is a zinc electrode that undergoes oxidation, while the cathode is a copper electrode that undergoes reduction. The construction of the cell involves two half-cells connected internally by a salt bridge, which maintains electrical neutrality by allowing ions to move between the half-cells.
The standard cell potential (Ecell) for the Daniell cell is 1.1 V when the concentration of both ions is 1 mol/dm³. The section illustrates the cell's operation under various external potentials, highlighting that at the standard potential, there is no net current flow. If an opposing voltage is applied equal to the Ecell, the cell operation ceases, and no reaction occurs, demonstrating the principles of electrochemical cells including the concepts of anode, cathode, and the movement of electrons.
Further, the relationship between cell potential and Gibbs free energy is explored, including the derivation of the Nernst equation, which provides insights into how concentrations of reactants impact cell potential. This section emphasizes the importance of understanding these cells for practical applications, such as batteries and electrochemistry in science and industry.
Youtube Videos







Audio Book
Dive deep into the subject with an immersive audiobook experience.
Introduction to Daniell Cell
Chapter 1 of 4
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
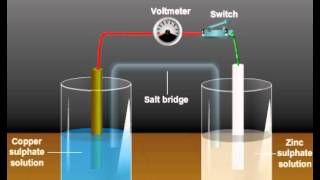
We had studied the construction and functioning of Daniell cell (Fig. 2.1). This cell converts the chemical energy liberated during the redox reaction Zn(s) + Cu2+(aq) ⇌ Zn2+(aq) + Cu(s) to electrical energy and has an electrical potential equal to 1.1 V when concentration of Zn2+ and Cu2+ ions is unity (1 mol dm–3). Such a device is called a galvanic or a voltaic cell.
Detailed Explanation
The Daniell cell is a type of galvanic cell, which means it generates electrical energy from spontaneous chemical reactions. It operates on the principle of redox reactions where zinc is oxidized and copper ions are reduced. In a simplified way, when zinc reacts with copper ions in a solution, energy is released, allowing the cell to produce an electric current. When the concentrations of Zn2+ and Cu2+ ions are both 1 mol/dm³, the cell can generate a voltage of 1.1 volts.
Examples & Analogies
Consider a Daniell cell like a water wheel in a river. Just as the water's flow (like a chemical reaction) turns the wheel, producing energy, the chemical reactions in the Daniell cell produce electrical energy. When both chemical reactants are present in the right amounts, the cell operates efficiently, similar to how a water wheel turns smoothly when there is a consistent flow of water.
Functioning under External Voltage
Chapter 2 of 4
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
If an external opposite potential is applied in the galvanic cell and increased slowly, we find that the reaction continues to take place till the opposing voltage reaches the value 1.1 V when the reaction stops altogether and no current flows through the cell. Any further increase in the external potential again starts the reaction but in the opposite direction.
Detailed Explanation
When you apply an external voltage to the Daniell cell, it can affect the spontaneity of the chemical reactions. As you increase the opposing voltage, the reactions inside the cell that produce electric current will continue until the opposing voltage reaches 1.1 volts. Beyond this point, the reactions stop, and no current flows because the applied voltage is greater than the maximum produced by the cell. If the voltage is increased even more, the cell can operate in reverse, using electricity to drive a reaction that normally would not occur spontaneously.
Examples & Analogies
Imagine pushing a swing. If you push with enough force, the swing will move higher until it reaches its peak (like reaching 1.1 V). If you push harder, the swing could start to go backward (the reverse reaction), but this requires more energy than what the swing can naturally achieve on its own.
Comparison with Electrolytic Cell
Chapter 3 of 4
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
When E > 1.1 V the cell functions as an electrolytic cell, a device for using electrical energy to carry non-spontaneous chemical reactions.
Detailed Explanation
When the applied voltage exceeds 1.1 V, the Daniell cell switches from generating electricity to consuming electricity, effectively acting as an electrolytic cell. In electrolytic cells, electrical energy from an external source is used to drive chemical reactions that would not typically occur on their own, allowing for processes like electroplating or the production of chemicals through electrolysis.
Examples & Analogies
Think of an electrolytic cell like a treadmill. While running (using energy) can generate momentum and help you move, when you jump onto a treadmill set to a steep incline, you’re using energy to move against gravity and change your position—it’s a direct application of energy to achieve a task (like an electrolytic process).
Daniell Cell Diagram
Chapter 4 of 4
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Daniell Cell diagram illustrates electrodes of zinc and copper dipping in their respective salt solutions (Fig. 2.1).
Detailed Explanation
The diagram of the Daniell cell shows two electrodes: a zinc electrode (anode) and a copper electrode (cathode) immersed in solutions of their respective sulfate salts (ZnSO₄ and CuSO₄). This visual representation is crucial for understanding how the cell is set up, as it highlights the flow of electrons and the movement of ions between the half-cells.
Examples & Analogies
Visualize the Daniell cell setup as two buckets connected by a pipe. One bucket filled with saltwater represents the Zn half-cell where zinc is gradually added, while the other bucket has copper saltwater, highlighting how water (like ions) flows and connects the two systems through the pipe (the salt bridge), allowing for the exchange necessary to produce electric energy.
Key Concepts
-
Electrochemical Cell: A device that converts chemical energy into electrical energy via redox reactions.
-
Redox Reaction: A chemical process involving the transfer of electrons between two species.
-
Standard Electrode Potential: The measure of individual electrode's ability to be reduced or oxidized under standard conditions.
-
Nernst Equation: A formula that relates the cell potential to the concentration of the reactants and products.
-
Conductivity: A measure of how well a solution can conduct electricity.
Examples & Applications
Example of a Daniell cell includes the reaction of zinc and copper sulfate solutions to generate electrical energy.
Real-life applications of Daniell cells can be seen in batteries, which use similar principles to store and provide electrical energy.
Memory Aids
Interactive tools to help you remember key concepts
Rhymes
In a Daniell cell, with Zn and Cu, oxidation, reduction, electricity’s the due.
Stories
Imagine a zinc rod in a salty lake with copper coins where the zinc gives away its 'family' of electrons to make power for a toy.
Memory Tools
Use 'ANK' to recall: Anode - Negatively charged during oxidation in a galvanic cell.
Acronyms
E = Zn - Cu to remember how to calculate cell potential using concentration.
Flash Cards
Glossary
- Daniell Cell
A type of galvanic cell that converts chemical energy into electrical energy through spontaneous redox reactions.
- Anode
The electrode where oxidation occurs in an electrochemical cell.
- Cathode
The electrode where reduction occurs in an electrochemical cell.
- Halfcell
A part of the electrochemical cell that contains either the oxidation or reduction reaction.
- Salt Bridge
A device in a galvanic cell that maintains electrical neutrality by allowing ions to flow between the half-cells.
Reference links
Supplementary resources to enhance your learning experience.
