Environmental Quality: Monitoring and Analysis
Enroll to start learning
You’ve not yet enrolled in this course. Please enroll for free to listen to audio lessons, classroom podcasts and take practice test.
Interactive Audio Lesson
Listen to a student-teacher conversation explaining the topic in a relatable way.
Introduction to Sediment-Water Systems
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Today, we will investigate how pollutants interact with sediment in water systems. Let's start by discussing what happens when a dense non-aqueous phase liquid, or D-NAPL, is released into a body of water.

What is a D-NAPL exactly, and how does it behave in water?

Good question! A D-NAPL is a contaminant that is denser than water, which causes it to sink. In contrast, a light non-aqueous phase liquid, or L-NAPL, is less dense and will float.

So D-NAPLs sink, but what happens when they reach the sediment?

When D-NAPLs reach the sediment, they can cause contamination as they dissolve or remain at the surface due to interfacial forces. This leads to chemical migration processes that we must carefully monitor.

What are the primary ways these contaminants spread?

Great follow-up! Contaminants can spread primarily through dissolution into the water, and through diffusion into the sediment, though they may also attempt to percolate through small pores, which can be quite challenging.

How do we measure the contamination levels in the sediment over time?

We use flux analysis at the sediment-water interface, where we evaluate how concentrations change over time due to various processes, including diffusion, desorption, and accumulation of contaminants.

To summarize, understanding D-NAPLs and L-NAPLs behavior in sediment-water systems is vital for predicting contamination spread and effective remediation strategies.
The Nature of Contaminants: D-NAPLs vs L-NAPLs
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Let's delve deeper into the differences between D-NAPLs and L-NAPLs. Can anyone recall what these acronyms stand for?

D-NAPL means dense non-aqueous phase liquid, and L-NAPL means light non-aqueous phase liquid.

Exactly! Now, why are these distinctions important?

Because they behave differently in the environment and affect how we think about remediation.

That's right! Since D-NAPLs sink, they can accumulate in sediments, while L-NAPLs tend to stay at the water's surface. This difference affects recovery methods.

How does sediment play a role in how we manage these contaminants?

Sediment is critical because it can act as a barrier or a reservoir for contaminants, influencing both the rate and total amount of contaminant that can enter the aquatic environment.

In summary, knowing how D-NAPLs and L-NAPLs interact with sediments helps us understand contamination profiles and develop effective monitoring.
Contamination Dynamics and Monitoring
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Now, let’s talk about monitoring contamination and the challenges involved. What challenges do we face with historically contaminated sites?

It takes time for contamination to spread, so by the time we realize there's a problem, it may be very dispersed.

Exactly! This delayed recognition of contamination makes remediation efforts challenging, especially if the responsible parties are no longer around.

What steps can we take to monitor these contaminants effectively?

Monitoring involves sampling both water and sediment over time to evaluate contaminant concentration, as well as understanding the underlying mass transfer dynamics at work here.

What about using models to predict contaminant behavior?

Good point! We can utilize mathematical and conceptual models to predict contaminant transport, which helps us in planning appropriate remediation strategies.

To conclude, effective monitoring relies on understanding both the historical context and the dynamics of contaminants at play.
Introduction & Overview
Read summaries of the section's main ideas at different levels of detail.
Quick Overview
Standard
The section delves into how contaminants like dense NAPLs (D-NAPLs) and light NAPLs (L-NAPLs) interact with water and sediment, highlighting their behaviors, such as sinking or floating, and the processes of dissolution and diffusion. It emphasizes the complexities of monitoring historical contamination, the challenges in sediment analysis, and the need for understanding mass transfer mechanisms in environmental engineering.
Detailed
Environmental Quality: Monitoring and Analysis
In-depth Summary
This section provides a comprehensive overview of how sediment and water interact in terms of contamination and transport mechanisms. It specifically focuses on the behavior of two types of non-aqueous phase liquids (NAPLs): dense NAPLs (D-NAPLs) and light NAPLs (L-NAPLs). D-NAPLs are characterized by their higher density than water, leading them to sink and interact differently with sediment compared to L-NAPLs, which float.
Key concepts include:
- Dissolution and Diffusion: The section explains how contaminants can dissolve in water and diffuse into sediment, creating plumes that can spread over time. This process is governed by mass transfer rates and can be influenced by pore structure and surface tension.
- Contaminated Sediment Modeling: Historical contamination poses challenges due to prolonged effects on both sediment and aquatic systems. An understanding of sediment-water interface dynamics is critical for predicting contaminant movement and developing remediation strategies.
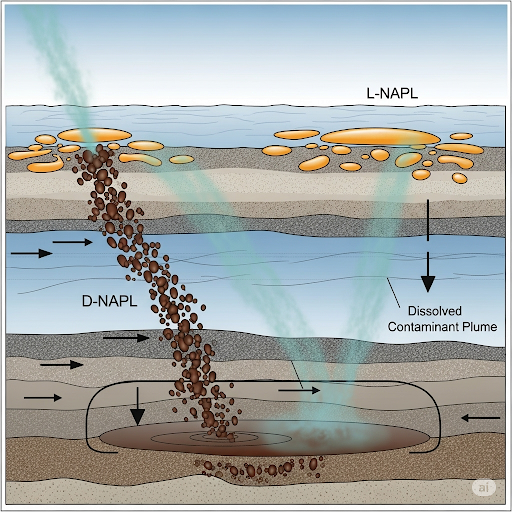
- Flux Analysis: The text details how to model the flux of contaminants at the sediment-water interface, factoring in concentration gradations and the complexities arising from diffusion and desorption processes.
These principles are essential for environmental engineers and scientists to assess and remediate contaminated sites effectively, with historical liabilities often complicating clean-up efforts.
Youtube Videos










Audio Book
Dive deep into the subject with an immersive audiobook experience.
Introduction to Sediment and Fluid Phases
Chapter 1 of 6
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
So, specifically what we are interested in is this system where there is a sediment. One is a solid phase, the other one is a fluid phase. So, it is sediment, water or soil, air systems. So, both of them are somewhat similar, but we will start with sediment water, it is the simplest system in terms of what happens.
Detailed Explanation
In this section, we discuss the interaction between sediment (solid phase) and water (fluid phase). This system is important for understanding how pollutants behave when they come into contact with sediments in water bodies. Sediments serve as a critical component in environmental quality, influencing how contaminants are transported and transformed in aquatic environments.
Examples & Analogies
Imagine a sponge soaked in water. The sponge represents sediment, while the water represents the fluid phase. Just as the sponge can absorb water and pollutants, sediments can interact with chemicals in the water, affecting their movement and concentration.
D-NAPL and L-NAPL Types
Chapter 2 of 6
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
So, these are what is called as dense NAPL or dense non-aqueous phase liquids. D-NAPL are those chemicals which are dense and then there are L-NAPL which are light. So, again as we discussed earlier, in D-NAPL the density is greater than the density of water, for LNAPL, here the density is less than the density of water.
Detailed Explanation
Dense Non-Aqueous Phase Liquids (D-NAPL) are heavier than water and sink to the bottom, while Light Non-Aqueous Phase Liquids (L-NAPL) float on the surface. This affects how contaminants behave in water systems, as D-NAPL can settle in sediments where they can persist and affect long-term environmental health.
Examples & Analogies
Consider oil and vinegar in a salad dressing. Oil floats on the surface (like L-NAPL), while vinegar is denser and sinks. In the same way, pollutants can behave differently based on their densities when they enter a water body.
Dissolution and Percolation Mechanisms
Chapter 3 of 6
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
When it enters here, one of the things that does happen to the sinkers, is that the dissolution starts taking place straightaway. Water is flowing, away, but it is also traveling inside, because there is a gradient. So, how does it go inside, is based on two mechanisms.
Detailed Explanation
When D-NAPL enters water and sediment, it can dissolve into the water quickly. This dissolution is driven by concentration gradients, causing the pollutant to mix into the water. Percolation, the movement of liquids through the sediment, can also occur; however, it is often impeded by surface tension and the structure of the sediment.
Examples & Analogies
Think about how sugar dissolves in water. When you stir sugar into a glass of water, it spreads out. Similarly, the dissolved pollutants spread in the water. However, not all solid substances dissolve easily, just like salt might take longer to dissolve in cold water.
The Concept of Plume Formation
Chapter 4 of 6
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Over a period of time, what can happen is you start with this big spill on the surface and over a period of time, this spill can spread. This marks the boundary of the chemical concentration, as it is similar to the plume we have in the atmospheric dispersion.
Detailed Explanation
As contaminants dissolve, they spread out in the water, forming what is known as a 'plume'. Similar to how smoke from a fire spreads into the air, when pollutants dissolve, they create a cloud of chemicals that can be detected over a larger area, marking a zone of higher concentration.
Examples & Analogies
Imagine dropping a few drops of food coloring in water. Initially, it is concentrated but over time, the color spreads out, creating a plume of colored water. This is similar to how dissolved contaminants spread in water.
Historical Contamination and Liability Issues
Chapter 5 of 6
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
This is the reason why we call it as historically contaminated sediment and these things have a contaminated site.
Detailed Explanation
Contaminated sediments from past spills can pose long-term environmental dangers, often lasting decades. These historical contaminations can lead to liability issues, as contemporary entities may be responsible for clean-up due to actions taken long ago, potentially complicating these issues with corporate history and responsibility.
Examples & Analogies
Think of a factory that dumped chemicals into a river decades ago. Now, people living downstream are affected. This historical contamination makes it difficult to pinpoint responsibility, as the company might no longer exist.
Understanding Flux and Concentration in Sediment-Water Interface
Chapter 6 of 6
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
When we invoke the word history, it means that very long back, we are saying 2 decades, 3 decades and all that. So the consequence is that sometimes when something may have been contaminated 30-40 years back and it is still there and it is causing an effect now.
Detailed Explanation
The concept of flux at the sediment-water interface refers to how fast contaminants move between sediment and water. Understanding this helps us predict pollution levels over time and can inform strategies for remediation or clean-up.
Examples & Analogies
Imagine water slowly seeping through a sponge. The speed at which water flows out of the sponge into the surrounding area is analogous to the flux of contaminants at a sediment-water interface. It highlights how important it is to monitor and manage contaminant levels.
Key Concepts
-
Sediment-Water Interface: The boundary where sediment meets water, critical for understanding chemical interactions and contaminant transport.
-
Concentration Gradient: The variation in chemical concentration that drives diffusion and can result in contaminant movement.
-
Contaminant Plumes: Areas of dissolved contaminants spreading through water or sediment, impacting ecological health.
Examples & Applications
Example of D-NAPL: Trichloroethylene, a common industrial solvent, which is denser than water and can cause significant groundwater contamination when spilled.
Example of L-NAPL: Petroleum products that float on water bodies, leading to surface water contamination and affecting local wildlife.
Memory Aids
Interactive tools to help you remember key concepts
Rhymes
D-NAPL sinks; L-NAPL floats, understanding this keeps us on correct notes.
Stories
Once in a river, a heavy oil sank. It started to spread, overcoming the bank. A fish swam by, unaware of the plight; it swam into pollution, no end in sight.
Memory Tools
SPLASH - Sediment, Permeation, LNAPL, Adsorption, Surface tension, Hydrodynamic gradient. Remember the concepts affecting sediment interactions.
Acronyms
NAPL
Non-aqueous Phase Liquid; remember
it's 'Not A Pure Liquid' in nature.
Flash Cards
Glossary
- DNAPL
Dense non-aqueous phase liquid; a type of contaminant that is denser than water and sinks in aquatic environments.
- LNAPL
Light non-aqueous phase liquid; a type of contaminant that is less dense than water and floats on the surface.
- Flux
The rate at which a substance moves through a given area, particularly in the context of contaminant transfer at the sediment-water interface.
- Dissolution
The process by which a solute (such as a contaminant) dissolves in a solvent (like water).
- Diffusion
The movement of particles from an area of higher concentration to an area of lower concentration.
Reference links
Supplementary resources to enhance your learning experience.
