Mass Transfer in the Environment
Enroll to start learning
You’ve not yet enrolled in this course. Please enroll for free to listen to audio lessons, classroom podcasts and take practice test.
Interactive Audio Lesson
Listen to a student-teacher conversation explaining the topic in a relatable way.
Introduction to Mass Transfer in Sediments
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Today, we will discuss mass transfer in sediment-water environments. Can anyone tell me what NAPLs are and why they are important?

NAPLs stands for non-aqueous phase liquids, right? They don't mix with water.

Exactly, Student_1! NAPLs can be dense or light; do you remember how we differentiate them?

Yeah, D-NAPLs sink in water, while L-NAPLs float above the water surface.

Great! Now, let's remember these with this acronym: D-NAPLs are 'Difficult to handle' because they sink into sediments, and L-NAPLs are 'Light and Lost' as they float. This will help you during exams!

How do these liquids affect the sediment over time?

Good question! D-NAPLs undergo dissolution initially, forming dissolved plumes. But it can take a long time for these chemicals to spread through sediment due to the resistance from pore water.

So, if D-NAPLs dissolve, then does it mean they are removed from contamination?

Not really, Student_4! Although they dissolve, they can still lead to contaminated sediments. Understanding this process is key to environmental monitoring.

In summary, today we learned about NAPLs and their behavior in sediments, plus some memory aids to help you recall critical points!
Percolation and Surface Tension
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Now, let’s discuss how surface tension influences the movement of D-NAPLs in sediment. Who can tell me what surface tension does?

Surface tension creates resistance, which makes it hard for liquids to pass through tiny openings.

Exactly! In fact, this surface tension can prevent D-NAPLs from percolating into sediment. What potential problem could this cause?

If they can't percolate, they might just sit on the surface, causing ongoing contamination.

Right! They may remain on the pore surfaces or slowly dissolve into water and form a plume instead. How do you think this impacts cleanup efforts?

It would make cleanup much more difficult and time-consuming if they don’t percolate!

Excellent point! In summary, we identified how surface tension plays a crucial role in mass transfer processes and cleanup challenges, taking note of lasting impacts.
Dissolution and Environmental Impacts
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Let’s talk about how D-NAPLs dissolve and diffuse. Can anyone explain the dissolution process?

Dissolution occurs when a solute dissolves in water and spreads out from the source.

Spot on! This spreading forms a concentration plume. What happens to this plume over time?

The concentration would gradually reduce as it diffuses away from the source.

Exactly! Now, thinking ahead, what can be the long-term effects of this dissolution on the environment?

If the chemicals accumulate downstream in fish or sediment, it could harm the ecosystem.

Great observation! Understanding the timeline for contamination is important for environmental responsibility. To wrap up, today’s lesson highlighted how dissolution can lead to broader ecological impacts.
Introduction & Overview
Read summaries of the section's main ideas at different levels of detail.
Quick Overview
Standard
The section delves into the complexities of mass transfer in environments where solid and fluid phases coexist, shedding light on the dissolution and diffusion mechanisms of dense and light non-aqueous phase liquids (D-NAPLs and L-NAPLs) in sediment-water systems. It emphasizes the importance of understanding these processes for assessing and remediating contaminated sites.
Detailed
Detailed Overview of Mass Transfer in Sediments
In this section, we explore the application of mass transfer theory to sediment-water systems, particularly in the context of environmental contamination. Understanding mass transfer is crucial for evaluating the fate and transport of pollutants, specifically non-aqueous phase liquids (NAPLs).
Key Concepts:
- Non-Aqueous Phase Liquids (NAPLs): These are liquids that do not mix with water, crucially categorized into dense non-aqueous phase liquids (D-NAPLs), which sink, and light non-aqueous phase liquids (L-NAPLs), which float on water.
- Dissolution and Percolation: When D-NAPLs spill on sediments, they start dissolving in water immediately; however, percolation into the sediment pore spaces is resisted by surface tension and the structure of sediments.
- Dissolution Process: The chemical spreads mainly through dissolution, forming a plume of dissolved materials. The plume represents diminishing concentrations over time.
- Contaminated Sites: The section discusses the challenges associated with historically contaminated sediments and the long-term effects of slow dissolution and diffusion processes.
- Modeling Flux: We delve into flux modeling at the sediment-water interface, key to understanding contaminant transport and assessing environmental liabilities.
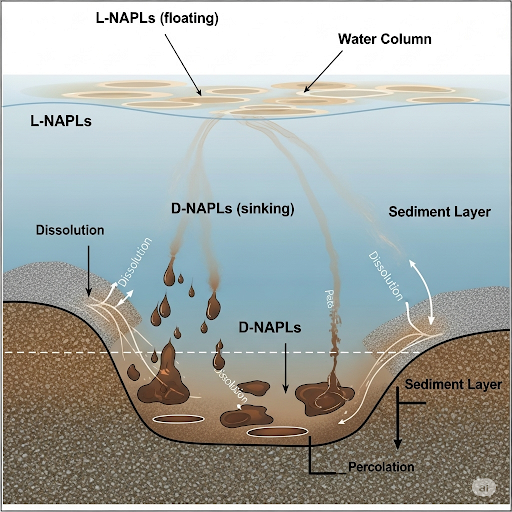
This knowledge is vital in the context of environmental engineering and monitoring, underscoring that sediment contamination can persist for decades, impacting ecological and human health.
Youtube Videos










Audio Book
Dive deep into the subject with an immersive audiobook experience.
Introduction to Mass Transfer in Sediments
Chapter 1 of 6
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Environmental systems consist of solid phases such as sediment in contact with fluid phases like water. The interaction is key to understanding mass transfer in the environment, especially in relation to contaminants.
Detailed Explanation
In environmental science, mass transfer refers to the movement of substances (such as pollutants) from one phase to another, which is essential for understanding how contaminants behave in natural systems. When looking at sediment and water systems, it's important to grasp how contaminants can migrate through these different phases.
Examples & Analogies
Imagine a spill of colored dye in a swimming pool. Initially, the dye might float on the surface or sink to the bottom, depending on its properties. Over time, the dye spreads throughout the water, similar to how contaminants in sediment can dissolve and mix with water.
Types of NAPL in Environmental Context
Chapter 2 of 6
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Contaminants can be non-aqueous phase liquids (NAPLs), which are categorized into dense naphthalene liquids (D-NAPLs) and light non-aqueous phase liquids (L-NAPLs). D-NAPLs sink in water, while L-NAPLs float.
Detailed Explanation
Non-aqueous phase liquids (NAPLs) are pollutants that do not dissolve well in water. D-NAPLs are denser than water and sink, making them particularly challenging to manage because they can settle deep in sediment layers. Conversely, L-NAPLs are less dense and float on the water's surface, leading to different environmental behaviors and remediation strategies.
Examples & Analogies
Think of oil on water. When oil spills into the ocean, it floats on the surface (L-NAPL), but if you pour a heavier liquid like honey into a glass of water, the honey sinks to the bottom (D-NAPL). This difference in behavior affects how we clean up these pollutants.
Interaction of D-NAPL with Sediment and Water
Chapter 3 of 6
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
When D-NAPL enters a sediment-water system, dissolution can occur immediately as water flows and moves through the sediment. However, the movement into the sediment is hampered by factors such as pore size and surface tension.
Detailed Explanation
When a dense pollutant (D-NAPL) sinks into a sediment area, it can dissolve into the water but faces challenges in entering the sediment due to the small size of sediment pores and the high surface tension of water. This means the D-NAPL might stay at the surface instead of penetrating deeply into the sediment. The process of dissolution is a mass transfer phenomenon that plays a critical role in how the contaminant spreads.
Examples & Analogies
Imagine trying to pour a thick syrup into a tiny bottle. The syrup may sit on the top instead of flowing in due to its viscosity. Similarly, contaminants can struggle to move through fine sediment layers, affecting how quickly they mix into the water.
Dissolution and Diffusion Processes
Chapter 4 of 6
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Once the D-NAPL starts dissolving, it spreads downward through the water, creating a plume of dissolved concentrations over time as the pollutant dissipates into the water column.
Detailed Explanation
As a D-NAPL dissolves in the water, it creates a plume or a concentration gradient that spreads out over time. This is similar to how a drop of food coloring disperses in a glass of water. The spread of the contaminant can be slow, depending on various factors such as temperature, water movement, and sediment characteristics.
Examples & Analogies
Think of putting a drop of ink in a glass of water. Initially, it remains concentrated, but gradually, it starts spreading out to create a larger area of colored water. This illustrates how contaminants can diffuse in water after dissolving.
Historical Contamination and Legal Implications
Chapter 5 of 6
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Contaminated sediments can be complex to manage due to historical pollution events, leading to long-lasting effects and liability issues. Over time, the identification of responsible parties becomes challenging.
Detailed Explanation
Historically contaminated sites pose significant challenges for environmental cleanup. If pollution occurred decades ago, it may be difficult to determine which organization or individual is responsible for the cleanup. These lingering contaminants can impact ecosystems and public health, often requiring extensive legal and regulatory efforts to address.
Examples & Analogies
Imagine a factory from 30 years ago that dumped waste into a river. Today, the river shows signs of contamination, but the factory may no longer exist. Identifying liability for cleanup becomes tough, just like figuring out who should fix a mess left by someone who is no longer around.
Modeling Contaminant Flux at Sediment-Water Interface
Chapter 6 of 6
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
To understand how contaminants move from sediment to water, scientists model flux at the sediment-water interface, defining key parameters such as concentration gradients.
Detailed Explanation
Modeling the flux of contaminants at the sediment-water interface helps scientists predict how quickly and in what manner pollutants will move from sediment into the water column. This involves measuring concentrations at different depths and understanding how these concentrations change over time as contaminants disperse.
Examples & Analogies
Think about measuring how quickly sugar dissolves in tea. By sampling the tea at various points in time and at different depths, you can create a model of how the sugar concentration increases over time. Similarly, environmental scientists use models to track pollutant concentrations in sediment and water.
Key Concepts
-
Non-Aqueous Phase Liquids (NAPLs): These are liquids that do not mix with water, crucially categorized into dense non-aqueous phase liquids (D-NAPLs), which sink, and light non-aqueous phase liquids (L-NAPLs), which float on water.
-
Dissolution and Percolation: When D-NAPLs spill on sediments, they start dissolving in water immediately; however, percolation into the sediment pore spaces is resisted by surface tension and the structure of sediments.
-
Dissolution Process: The chemical spreads mainly through dissolution, forming a plume of dissolved materials. The plume represents diminishing concentrations over time.
-
Contaminated Sites: The section discusses the challenges associated with historically contaminated sediments and the long-term effects of slow dissolution and diffusion processes.
-
Modeling Flux: We delve into flux modeling at the sediment-water interface, key to understanding contaminant transport and assessing environmental liabilities.
-

-
This knowledge is vital in the context of environmental engineering and monitoring, underscoring that sediment contamination can persist for decades, impacting ecological and human health.
Examples & Applications
An oil spill in a river can lead to D-NAPL sinking into the sediment and gradually dissolving into the water, creating a plume.
In an industrial accident, a toxic chemical that sinks into sediments may lead to long-term contamination affecting aquatic life.
Memory Aids
Interactive tools to help you remember key concepts
Rhymes
D-NAPL sinks, like stones in streams, L-NAPL floats on water's gleams.
Stories
Imagine dropping two types of liquids in water: one sinks to the bottom while the other floats. This scenario illustrates how NAPLs behave in the environment!
Memory Tools
Remember 'D for Down' (D-NAPL) and 'L for Lift' (L-NAPL) to recall their behavior in water.
Acronyms
Learn to use D-NAPL and L-NAPL
SINK vs FLOAT helps remember their nature.
Flash Cards
Glossary
- NonAqueous Phase Liquid (NAPL)
A liquid that does not mix with water; can be dense (D-NAPL) or light (L-NAPL).
- DNAPL
Dense non-aqueous phase liquid, which has a density greater than that of water and sinks in it.
- LNAPL
Light non-aqueous phase liquid, which has a density less than that of water and floats on it.
- Percolation
The process of a liquid moving through a porous material.
- Dissolution
The process where a solid, liquid, or gas forms a solution in a solvent.
- Diffusion
The movement of particles from an area of high concentration to an area of low concentration.
- Plume
A body of fluid that shows a concentration gradient in space and time, often as a result of pollutant dispersion.
Reference links
Supplementary resources to enhance your learning experience.
