Flux Calculations at the Sediment-Water Interface
Enroll to start learning
You’ve not yet enrolled in this course. Please enroll for free to listen to audio lessons, classroom podcasts and take practice test.
Interactive Audio Lesson
Listen to a student-teacher conversation explaining the topic in a relatable way.
Understanding NAPLs
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Today, we are diving deep into the concept of Non-Aqueous Phase Liquids or NAPLs. Can anyone tell me the difference between D-NAPL and L-NAPL?

D-NAPL is a dense non-aqueous liquid that sinks, while L-NAPL is light and floats on water.

Correct! Remember, D-NAPL stands for Dense-NAPL which settles on sediment surfaces, while L-NAPL floats. A quick way to remember this is: 'D for Densely Sinking.' Now, what challenges do you think D-NAPL faces when entering a sediment?

It might struggle to penetrate the sediment due to the tight pores and surface tension.

Exactly! High surface tension creates resistance for D-NAPL. This is crucial for understanding their behavior. Now, let's summarize: What are the key characteristics of D-NAPL and its implications?

D-NAPL sinks and has a harder time penetrating sediments due to surface tension.

Well done! Understanding the behavior of NAPLs is essential in environmental quality monitoring.
Dissolution and Diffusion Mechanisms
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Now, let’s dive into the processes of dissolution and diffusion. What happens when a D-NAPL contaminates the sediment-water interface?

The D-NAPL dissolves in the water and spreads out as a plume, right?

Correct! The plume represents the diluted concentration of contaminants. What do you think governs how fast this dissolution occurs?

It must depend on the concentration gradients and temperature.

Also, the surface area of the D-NAPL exposed to water!

Yes! Both concentration gradients and surface area are key factors. Always remember the mnemonic 'DISSOLVE': Diffusion Increases Surface-area Supporting One Liquid's Volume Expansion. Rethink dissolution dynamics considering these factors.

That's a catchy way to remember it!

To conclude this session, what are the main points we've learned about dissolution and diffusion?

D-NAPLs dissolve based on concentration gradients and surface area, leading to plume formation.
Modeling Fluxes
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Let’s shift gears and talk about modeling the flux at the sediment-water interface. When we calculate the flux, what key factors do we consider?

We need to look at the concentration of contaminants at the sediment and compare it to the background concentration in the water.

Absolutely! This leads to calculating the mass transfer coefficient as well. Can someone explain the equation for flux?

I think it’s Flux (𝑛) equals the mass transfer coefficient times the difference in concentration at the interface and the background concentration!

Right! It's denoted as 𝑛 = 𝑘 × (𝜌| − 𝜌∞). When pondering flux during contamination, remember that unsteady states are often at play. What does that mean?

It means the flux isn’t constant – it changes over time as contaminants diffuse and are absorbed!

Great insight! This unsteady nature is crucial for our understanding of historically contaminated sediments. Can someone provide an example of how historical contamination affects flux calculations?

If contamination happened decades ago, the concentration might have diminished, but the sediments still hold remnants which complicate cleanup efforts.

Exactly! Historical context adds layers of complexity to our models. Always consider the past when dealing with contamination. Let’s summarize: What is the equation and the key terms involved in flux modeling?
Introduction & Overview
Read summaries of the section's main ideas at different levels of detail.
Quick Overview
Standard
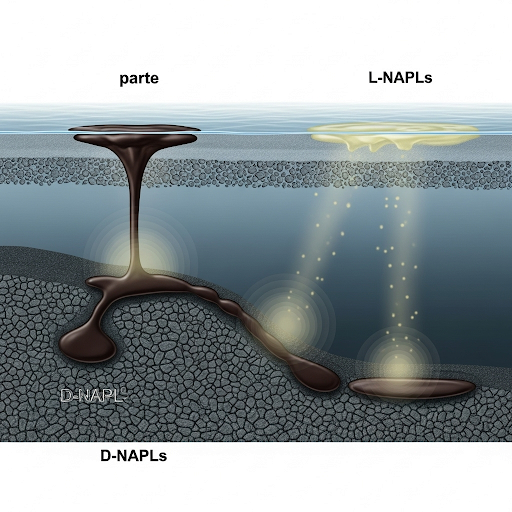
The section examines the interactions at the sediment-water interface, specifically regarding the fate of dense and light non-aqueous phase liquids (NAPLs) and the mechanisms of dissolution, diffusion, and percolation. The challenges of modeling fluxes in contaminated sediment scenarios are also discussed.
Detailed
Detailed Summary
This section explores the intricate interactions between sediments and water, specifically at the sediment-water interface where contamination events have significant implications. The discussion begins with an overview of non-aqueous phase liquids (NAPLs), categorized into dense (D-NAPL) and light (L-NAPL), based on their density relative to water. D-NAPLs sink and settle on sediments, while L-NAPLs float. The primary focus is how these NAPLs behave after a spillage, particularly how their dissolution and diffusion into water occur as a function of concentration gradients.
The sediment structure presents barriers for percolation due to surface tension and pore access issues, influencing the extent NAPLs can penetrate sediments. Over time, chemicals either spread as dissolved plumes or remain adsorbed at the sediment-water interface. Critical to understanding feedback loops at play, the flux of chemicals into the water is examined, emphasizing that this flux is inherently dynamic and depends on various transport mechanisms.
The section highlights the modeling challenges associated with calculating fluxes at contaminated sites, considering that historical contamination can lead to liability issues. As sediments hold and slowly release contaminants, their management necessitates an understanding of both transport dynamics and historical accountability, underlining the complexity of sediment-water interfaces in environmental quality monitoring.
Audio Book
Dive deep into the subject with an immersive audiobook experience.
Understanding D-NAPL and L-NAPL
Chapter 1 of 5
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
So, yesterday's class we looked at one example where there was a spillage of material. A chemical which sink down and lands on the surface of the sediment. So, these are what is called as dense NAPL or dense non-aqueous phase liquids. D-NAPL are those chemicals which are dense and then there are L-NAPL which are light. D NAPL are also called as sinkers and L NAPL are also called as floaters.
Detailed Explanation
In this chunk, we focus on two types of non-aqueous phase liquids (NAPLs): dense (D-NAPL) and light (L-NAPL). D-NAPL has a density greater than water, leading to it sinking and settling on the sediment layer. In contrast, L-NAPL, being less dense than water, floats. This distinction is crucial as it affects how these substances interact with their environment in terms of transportation and eventual dispersion. Understanding these properties helps us grasp the challenges in dealing with such contaminants in aquatic environments.
Examples & Analogies
Imagine pouring oil (L-NAPL) on top of water; it floats, forming a layer. Conversely, if you were to pour honey (D-NAPL) into water, it would sink straight to the bottom. Similarly, chemicals behave differently based on their densities, influencing how they move through water and sediment.
Dissolution vs. Percolation
Chapter 2 of 5
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
So when it enters here, one of the things that does happen to the sinkers is that the dissolution starts taking place straightaway. Water is flowing away, but it is also traveling inside, because there is a gradient. If percolation is possible, it will do percolation in porous medium. It is very hard especially in the presence of water in a pore provides a lot of resistance for displacement.
Detailed Explanation
This chunk discusses the processes that occur when a dense chemical contaminant sinks to the sediment-water interface. Initially, dissolution—the process where the contaminant dissolves into the water—occurs. However, percolation, or the movement of liquid through the sediment's pores, is often obstructed due to the resistance created by water's presence in these tiny spaces. This resistance means that, while some chemicals may dissolve, others struggle to penetrate deeper into the sediment, leading to different scenarios of contamination.
Examples & Analogies
Think of it like trying to pour syrup (D-NAPL) into a sponge soaked in water. The syrup may sit on top without easily absorbing into the sponge due to the water filling the sponge's pores, making it hard to push anything else into those spaces.
Contaminant Accumulation and Plumes
Chapter 3 of 5
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Over a period of time, this spill can spread. We are calling this a plume because it marks the boundary of the chemical concentration, similar to the plume we have in atmospheric dispersion. Now, here the boundary is a plume of dissolved concentrations.
Detailed Explanation
As time passes, the contamination from the D-NAPL spill spreads, leading to what is termed a 'plume.' This plume maps the concentration gradient of the dissolved contaminant in water over time. Just like smoke rising into the air, the way in which these chemicals spread illustrates the extent of contamination that can occur, emphasizing the slow process of dispersion in aquatic systems.
Examples & Analogies
Imagine dropping a drop of food coloring into a glass of water. Initially, the color is concentrated and localized; over time, it diffuses, creating a plume of colored water, illustrating how contaminants in water can spread over time.
The Importance of Historical Contamination
Chapter 4 of 5
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
When we invoke the word history, it means that very long back, we are saying 2 decades, 3 decades and all that. So the consequence is that sometimes when something may have been contaminated 30-40 years back and it is still there and it is causing an effect now.
Detailed Explanation
This section highlights the long-term impacts of historical contamination, where substances released decades ago can still affect present-day environments. Contaminated sites often face liability issues as the responsible parties may no longer exist, complicating cleanup efforts and accountability.
Examples & Analogies
Consider a situation where an old factory dumped waste into a river decades ago. Now, years later, fish in that river show high levels of toxins, leading to health issues for those who consume them. This shows how past actions can create long-lasting environmental impacts that require urgent attention now.
Modeling Flux at the Sediment-Water Interface
Chapter 5 of 5
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
How do you model this flux? We are interested in the flux at the surface. We are interested in flux into the water with an interface with the sediment. Now, how do you define this flux?
Detailed Explanation
Modeling the flux at the sediment-water interface involves understanding how contaminants move from the sediment into the water. The term 'flux' refers to the rate of flow of the contaminants through this interface. The challenge lies in accurately defining this flux, especially in the context of varying concentrations and effects on ecosystem health.
Examples & Analogies
Think of a sponge immersed in water. If you wring it out, the water (representing the contaminants) flows out of the sponge into the surrounding area. Similarly, understanding how contaminants are released from sediment into the surrounding water helps us assess environmental health and pollution.
Key Concepts
-
D-NAPL vs L-NAPL: D-NAPL sinks and L-NAPL floats, affecting how each contaminates the environment.
-
Dissolution & Diffusion: Concepts pivotal to understanding how contaminants spread in sediment-water interfaces.
-
Flux Dynamics: Flux is not constant and depends on concentration gradients and historical context.
Examples & Applications
A D-NAPL spill can create a plume in a river as it dissolves and spreads.
Historical contamination can lead to long-lasting effects in sediment, complicating clean-up efforts.
Memory Aids
Interactive tools to help you remember key concepts
Rhymes
In sediment deep and wide, D-NAPLs sink with pride.
Stories
Once, a dense liquid named D-NAPL wanted to see the world, but as it sank into the sediment, it learned that spreading meant adventure in dissolution.
Memory Tools
Remember to 'DISSOLVE': Diffusion Increases Surface-area Supporting One Liquid's Volume Expansion.
Acronyms
NAPL = Non-Aqueous Phase Liquid, to remember its properties.
Flash Cards
Glossary
- DNAPL
Dense Non-Aqueous Phase Liquid that sinks and settles on sediments.
- LNAPL
Light Non-Aqueous Phase Liquid that floats on water.
- Dissolution
The process through which a solid turns into a liquid solution.
- Flux
The rate of transfer of substances across a surface area.
- Contaminated Sediment
Sediment that has been polluted by hazardous substances.
- Plume
A body of contaminated water spreading from a source of pollution.
Reference links
Supplementary resources to enhance your learning experience.
