Modeling the System
Enroll to start learning
You’ve not yet enrolled in this course. Please enroll for free to listen to audio lessons, classroom podcasts and take practice test.
Interactive Audio Lesson
Listen to a student-teacher conversation explaining the topic in a relatable way.
Introduction to NAPLs
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

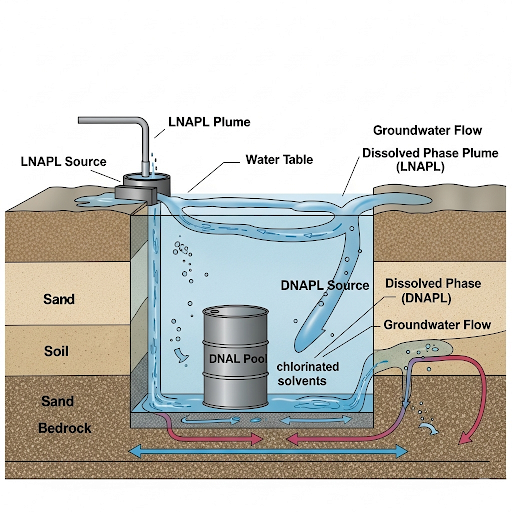
Today, we will learn about non-aqueous phase liquids, or NAPLs. There are two types: light NAPLs that float and dense NAPLs that sink. Can anyone tell me the differences?

D-NAPLs sink, and L-NAPLs float, right?

What happens when they spill into water?

Great questions! When D-NAPLs spill they can sink and potentially adhere to sediments, while L-NAPLs will float. Remember the acronym 'SINK' for D-NAPLs—'S' for sink, 'I' for insoluble, 'N' for non-aqueous, and 'K' for toxic!

What determines how far they spread?

The spread is primarily driven by dissolution and diffusion, which we will discuss further. Remember, they can also create contamination plumes in the water.
Mass Transfer Mechanisms
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Can anyone elaborate on how D-NAPLs interact with sediments?

They sink into the sediments!

But they don't just percolate in easily, right?

Exactly! Due to pore structure and surface tension, they primarily dissolve into the water, forming a plume. This process can be slow, and we often model it with the kinetics equation. Remember 'DISSOLVE' for understanding sediment interactions—'D' for dynamic, 'I' for interface, 'S' for surface tension, and so on!

Does groundwater flow affect this?

Yes, water flow significantly impacts how contaminants disperse!
Contamination and Cleanup Challenges
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Why do we often refer to historical contamination?

Because some pollutants stay in the environment a long time?

And it can be hard to trace who made the contamination!

Exactly! This is crucial for liability issues. The lingering presence of D-NAPLs creates ongoing contamination risks, especially when concentrations increase in aquatic life. Remember the phrase 'HIDDEN TIDES' for understanding this impact on ecology—'H' for hazardous, 'I' for invisible, 'D' for long-term, and so on!

So we need a good model for cleanup efforts?

Yes, exactly! Modeling helps to predict and manage contamination effectively.
Modeling Flux at Sediment-Water Interface
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Can someone explain what we mean by flux at the sediment-water interface?

Isn't it how much contaminant moves from the sediment into the water?

Exactly! The equation we use is based on concentrations at the boundary. Remember 'FLOW'—'F' for flux, 'L' for liquid phase, 'O' for outflow, and 'W' for water!

And that flux can change over time?

Yes, flux is time-dependent due to ongoing diffusion and changing concentration gradients!
Introduction & Overview
Read summaries of the section's main ideas at different levels of detail.
Quick Overview
Standard
The section covers the dynamics of contamination in sediment systems, focusing on the fate and transport of D-NAPLs and L-NAPLs. It explains how these contaminants interact with water and sediment, the complexities of dissolution and percolation, and the significance of equilibrium and mass transfer in modeling contaminated sediments.
Detailed
In this section, we explore the modeling of contamination in sediment systems, particularly through the interaction of dense non-aqueous phase liquids (D-NAPLs) and light non-aqueous phase liquids (L-NAPLs) with sediment and water. The discussion begins with the behavior of D-NAPLs, which are denser than water and tend to sink into sediments upon spillage, and contrasts this with L-NAPLs, which float on water. We examine how D-NAPLs primarily dissolve into the water rather than percolating through sediment due to high surface tension and pores' resistance. Over time, a plume of contamination can develop as dissolved chemicals spread through the water column. Modeling the flux of contaminants at the sediment-water interface is essential to understand the fate of these pollutants and the longer-term liabilities associated with historical contamination events.
Youtube Videos










Audio Book
Dive deep into the subject with an immersive audiobook experience.
Introduction to Sediment and Fluid Systems
Chapter 1 of 6
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
So, specifically what we are interested in is this system where there is a sediment. One is a solid phase, the other one is a fluid phase. So, it is sediment, water or soil, air systems. So, both of them are somewhat similar, but we will start with sediment water, it is the simplest system in terms of what happens.
Detailed Explanation
In this chunk, we introduce the concept of a sediment-water system, which involves a solid phase (the sediment) and a fluid phase (the water). The sediment can consist of materials like soil, while the fluid can be either water or air. The focus here is on how these two phases interact, with sediment-water being presented as the simplest system for discussion.
Examples & Analogies
Think of this as a sponge submerged in water. The sponge represents the sediment, and the water represents the fluid phase. This simple configuration allows us to explore how contaminants can move between the water and the sediment.
Types of Non-Aqueous Phase Liquids (NAPLs)
Chapter 2 of 6
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
So, in yesterday's class we looked at one example where there was a spillage of material. A chemical which sink down and lands on the surface of the sediment. So, these are what is called as dense NAPL or dense non-aqueous phase liquids.
Detailed Explanation
This part explains the types of NAPLs that can be involved in sediment contamination. Dense NAPLs (D-NAPLs) are those that sink and settle on the sediment's surface due to their higher density compared to water. Conversely, light NAPLs (L-NAPLs) float on the water's surface because they are less dense. Understanding these distinctions is crucial for analyzing how these substances behave in the environment.
Examples & Analogies
Imagine oil spilled on water. Oil is a light NAPL (L-NAPL) that floats, while a dense chemical like mercury would sink (D-NAPL). This behavior affects how these substances interact with the water and the sediment below.
Dissolution and Percolation Processes
Chapter 3 of 6
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
When it enters here, one of the things that does happen to the sinkers, is that the dissolution starts taking place straightaway. Water is flowing away, but it is also traveling inside, because there is a gradient.
Detailed Explanation
In this section, the processes of dissolution and percolation are discussed. When a D-NAPL contaminant sinks to the sediment, the surrounding water can dissolve some of the chemical immediately. The water is not just flowing away; it's also moving into the sediment due to concentration gradients. This flow can initially be resisted by surface tension within the sediment's pores, making percolation of the contaminant into the sediment challenging.
Examples & Analogies
Think of a sugar cube being dropped into a glass of water. As it dissolves, sugar spreads throughout the water, affecting the overall sweetness. Similarly, contaminants dissolve and spread from the sediment into the water, but sometimes they may not easily penetrate into the sediment.
Formation of Contaminated Sediment Plumes
Chapter 4 of 6
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Over a period of time, what can happen is you start with this big spill on the surface and over a period of time, this spill can spread. We are calling this a plume because it marks the boundary of the chemical concentration, as it is similar to the plume we have in the atmospheric dispersion.
Detailed Explanation
This chunk introduces the concept of a plume, which is the area where the concentration of contaminants can be detected. As the contaminant dissolves in the water and diffuses, the concentration spreads out, forming a plume. This process takes time and involves the gradual mixing of contaminants with water, similar to how smoke disperses in the air.
Examples & Analogies
Imagine throwing a handful of dye into a still pond. As the dye spreads, it forms a visible plume of color, marking the area of higher concentration. This is akin to how contaminants spread from a sediment source into the water.
Contaminated Sediment Dynamics Over Time
Chapter 5 of 6
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
When we invoke the word history, it means that very long back, we are saying 2 decades, 3 decades and all that. So the consequence is that sometimes when something may have been contaminated 30-40 years back and it is still there and it is causing an effect now...
Detailed Explanation
The focus of this section is on the historical aspects of sediment contamination. Many sites remain contaminated long after the original pollution occurred, sometimes stemming from incidents decades ago. These contaminants can continue to affect the environment, leading to ongoing legal and cleanup challenges as those responsible may no longer exist.
Examples & Analogies
Consider an old factory site that closed down decades ago but still has lingering toxic waste in the soil. Even if the factory has been demolished, the chemicals can persist in the environment, impacting local ecosystems and necessitating expensive cleanup efforts.
Modeling Flux at the Sediment-Water Interface
Chapter 6 of 6
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
So, under water, you cannot see. You think everything is fine. When will you see it? You will see it when there is a concentration in the water downstream...
Detailed Explanation
This part discusses the importance of modeling flux at the interface where sediment meets water. It emphasizes that monitoring contaminant concentration in water downstream is critical for detecting sediment contamination. The mathematical model for flux is introduced, showing how background concentration and the concentration at the interface influence the rates of flow and contaminant transport.
Examples & Analogies
Consider a sink draining water. Initially, you can’t see any gunk at the bottom, but over time, you notice some foul smells or discoloration in the water flowing away. This indicates contamination in the sediment that becomes evident through the water, just like how sediment contamination might only be noticed through downstream effects.
Key Concepts
-
D-NAPL: A dense contaminant that sinks in water and may stay at the sediment-water interface.
-
L-NAPL: A lighter contaminant that floats on water and behaves differently from D-NAPL.
-
Contamination Plume: A region of measured pollutant spreading through the water, representing the concentration gradient.
-
Mass Transfer: The process determining how contaminants move from sediments into water based on various mechanisms.
Examples & Applications
An oil spill scenario where D-NAPL sinks into sediment and creates a contaminant plume in the water.
Explaining how sediment thresholds affect L-NAPL dispersion versus D-NAPL.
Memory Aids
Interactive tools to help you remember key concepts
Rhymes
If it sinks, it's D-NAPL, so always remember, for spills, it's a hazard, like a dark winter!
Stories
Once upon a time, there were two types of spills in a lake—light ones floated like ducks, while heavy ones sank, polluting the sand.
Memory Tools
Remember 'SINK' for D-NAPL: 'S' for sink, 'I' for insoluble, 'N' for non-aqueous, 'K' for known risk.
Acronyms
Use 'FLOW' to recall about flux
'F' for flux
'L' for liquid phase
'O' for outflow
'W' for water.
Flash Cards
Glossary
- DNAPL
Dense Non-Aqueous Phase Liquid; substances denser than water that sink in aquatic environments.
- LNAPL
Light Non-Aqueous Phase Liquid; substances less dense than water that float on the surface.
- Plume
A spreading concentration of pollutants in water or air, often originating from a contamination source.
- Dissolution
Process of a solid, liquid, or gas dissolving in a solvent, often fundamental in contaminant transport.
- Percolation
Movement of water and dissolved substances through soil and pore spaces in sediments.
- Mass Transfer
The movement of mass from one location to another, affecting how contaminants spread.
Reference links
Supplementary resources to enhance your learning experience.
