Electron Configurations and Energy Levels
Enroll to start learning
You’ve not yet enrolled in this course. Please enroll for free to listen to audio lessons, classroom podcasts and take practice test.
Interactive Audio Lesson
Listen to a student-teacher conversation explaining the topic in a relatable way.
Early Atomic Models
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Let's start by discussing early atomic models, like Rutherford's and Bohr's. Rutherford's model showed that atoms are mostly empty space with a dense nucleus at the center. Can anyone explain why this was an important discovery?

It helped us understand that the positive charge and most of the mass in an atom are concentrated in a small area!

Exactly! Now, Bohr built on this by introducing quantized orbits for electrons. Can anyone tell me what quantized means?

It means the electrons can only exist in specific energy levels, not between them.

Great! So, while it worked for hydrogen well, Bohr's model didn't fit multi-electron atoms. This led to the quantum mechanical model, where we represent electrons in terms of probabilities instead of fixed paths.

So, instead of paths, we refer to regions called orbitals?

Yes! Let's summarize: Rutherford showed us about the nucleus, Bohr introduced energy levels, and together they set the stage for quantum mechanics. Understanding these models helps us see the bigger picture of electron behavior.
Quantum Mechanical Model
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Now let’s talk about the quantum mechanical model of the atom. This model uses wavefunctions to define where an electron is likely to be found. What are the quantum numbers we use to describe these states?

There are four: n, ℓ, m_ℓ, and m_s!

Correct! Each of these contributes to defining an electron's state. What about n? What does it indicate?

It indicates the principal energy level or shell of the electron.

Yes! And ℓ tells us about the subshells like s, p, d, and f. Can anyone explain the significance of m_ℓ?

It specifies the orientation of the orbital in space.

Exactly! Remember: the quantum mechanical model emphasizes probability over certainty. As we move through this section, let's keep in mind how each quantum number shapes our understanding of electron behavior.
Electron Configuration Rules
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Let's discuss the rules for filling orbital energy levels. The Aufbaus Principle states that electrons will occupy the lowest energy orbitals first. Why do you think that is important?

It helps maintain stability in the atom by minimizing energy!

Exactly! And then there's the Pauli Exclusion Principle: no two electrons can have the same set of four quantum numbers. Can someone give me an example?

If two electrons are in the same orbital, they need to have opposite spins!

Perfect! Now, Hund’s Rule tells us that when electrons occupy orbitals of equal energy, they fill singly first before pairing up. How does that minimize electron-electron repulsion?

By keeping them in separate orbitals, they have less chance of repelling each other!

Exactly! Let's recap: Aufbau fills from lowest energy, Pauli excludes identical quantum states, and Hund's arranges to minimize repulsion. Understanding these principles prepares us for drawing electron configurations for different elements.
Writing Electron Configurations
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Now that we understand the principles, let's write some electron configurations! What do we mean by standard notation?

It lists orbitals in increasing energy order, adding superscripts for how many electrons occupy each.

That's right! Can someone give me an example for carbon?

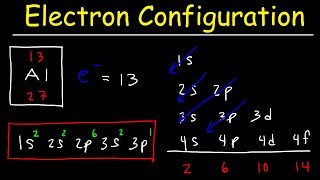
Carbon, which has 6 electrons, would be 1s² 2s² 2p².

Correct! Now, for larger elements, we often use noble gas core notation to simplify. Who can explain how that works?

You take the configuration of the nearest noble gas and put it in brackets, then add the remaining orbitals.

Exactly! For example, chlorine would be written as [Ne] 3s² 3p⁵. Let’s conclude this session by summarizing how to write both configurations and when to use each method.
Quantum Numbers and Orbital Shapes
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Finally, let’s look at how quantum numbers not only describe energy levels but also the shape of orbitals. Who can tell me about the shapes of s, p, and d orbitals?

S orbitals are spherical, p orbitals are dumbbell-shaped, and d orbitals have more complex cloverleaf shapes.

Correct! Each of these shapes affects how atoms bond. How might the shape of an orbital impact the chemical behavior of an atom?

The shape determines how closely they can approach other atoms and how bonds are formed.

Excellent! Remember, the arrangement of electrons and their distribution in these orbitals is key to understanding an atom’s reactivity. Let's summarize: Quantum numbers dictate both energy levels and shapes of orbitals, influencing chemical properties.
Introduction & Overview
Read summaries of the section's main ideas at different levels of detail.
Quick Overview
Standard
The arrangement of electrons is crucial for understanding atomic structure and chemical properties. This section delves into early atomic models, the quantum mechanical model, principles governing electron configurations, and notation methods, illustrating how these concepts are foundational for chemistry and physics.
Detailed
Electron Configurations and Energy Levels
Understanding how electrons are arranged in quantized energy levels around an atom's nucleus helps predict its chemical behavior. This section provides a comprehensive overview of various atomic models leading to the quantum mechanical model, as well as how to effectively represent electron configurations.
Key Topics Covered:
- Early Atomic Models:
- Rutherford's Nuclear Model: Described a nucleus containing most of an atom's mass and positive charge, with electrons orbiting around it.
- Bohr Model: Introduced quantized orbits to explain hydrogen's discrete energy levels and spectrum.
- Limitations of Bohr's Model: Lacked accuracy for multi-electron atoms and phenomena such as spin.
- Quantum Mechanical Model:
- Developed from De Broglie and Schrödinger's theories, it replaced fixed orbits with electron probability distributions called orbitals. The model uses quantum numbers to describe atomic orbitals and their shapes.
- Electron Configuration:
- Electrons fill energy levels from lowest to highest energy according to the Aufbau Principle, influenced by the Pauli Exclusion Principle and Hund's Rule.
- Notation methods include standard configurations and noble gas core notation, illustrating how electrons occupy orbitals.
- Energy Levels and Orbitals:
- Understanding principal quantum numbers (n), azimuthal quantum numbers (ℓ), and their implications for orbital shapes and orientations is essential.
- Significance:
- The arrangement of electrons is fundamental in explaining periodic trends, chemical bonding, and the reactivity of elements across the periodic table.
In studying this section, the student gains substantial insight into how atomic structure and electron configuration are intricately tied to chemical behavior.
Youtube Videos
![Electron Configurations [IB Chemistry SL/HL]](https://img.youtube.com/vi/u5w2NKm8ZPg/mqdefault.jpg)

Audio Book
Dive deep into the subject with an immersive audiobook experience.
Early Atomic Models
Chapter 1 of 6
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
2.1 Early Atomic Models: From Bohr to the Quantum Mechanical Model
2.1.1 Rutherford’s Nuclear Model (Post-1911)
- Rutherford’s experiments proved that:
- An atom’s positive charge and most of its mass are concentrated in a small, dense nucleus.
- Electrons move around this nucleus in otherwise empty space.
- Limitation: According to classical physics, an accelerating charged particle (like an electron in circular orbit) should continuously emit radiation, lose energy, and spiral into the nucleus. Yet atoms are stable; electrons do not collapse into the nucleus.
2.1.2 Bohr Model (1913)
Niels Bohr proposed a semi-classical model for the hydrogen atom (and other hydrogen-like ions) that successfully explained the stability of atoms and the discrete line spectrum of hydrogen:
1. Quantized Orbits: Electrons orbit the nucleus in circular orbits but do not emit radiation while in those orbits. Each allowed orbit corresponds to a fixed energy level “E sub n” (for n = 1, 2, 3, …).
2. Angular Momentum Quantization: Only orbits in which the electron’s angular momentum is an integer multiple of the reduced Planck constant (denoted “h-bar”) are allowed. That is, m × v × r = n × h-bar, where…
3. Energy of the n-th Level: For hydrogen (nucleus charge +1), the energy of an electron in the n-th orbit equals -13.6 electron-volts divided by n squared…
4. Photon Emission or Absorption: When the electron jumps from a higher level (nᵢ) to a lower level (n_f), it emits a photon whose energy equals the difference between the two levels: Energy of photon = Eᵢ – E_f.
5. Limitations of the Bohr Model: It accurately predicts hydrogen-like spectra (one-electron systems such as H, He⁺), but fails for multi-electron atoms.
Detailed Explanation
The early atomic models were foundational in understanding atomic structure. Rutherford’s model proposed that atoms have a dense nucleus with electrons orbiting around it, yet it could not account for the stability of these orbits due to energy loss. Bohr then refined this model by introducing quantized energy levels where electrons could only exist in specific orbits without radiating energy, posing that any energy change by an electron would result in the emission or absorption of a photon. This explained the discrete lines observed in atomic spectra.
Examples & Analogies
Think of a planet orbiting a sun. According to classical mechanics, as the planet (the electron) moves faster around the sun (the nucleus), it would eventually spiral inward. However, like certain stable 'orbits' that each planet has, electrons can only exist in stable 'orbits' that are quantized, similar to how satellites maintain specific distances from Earth without crashing down.
Quantum Mechanical Model
Chapter 2 of 6
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
2.1.3 Quantum Mechanical Model (Wave Mechanics)
Building on De Broglie’s matter waves and Schrödinger’s wave equation, the modern quantum mechanical model replaces fixed circular orbits with three-dimensional probability distributions known as orbitals:
1. De Broglie Hypothesis (1924): A particle of mass m moving at speed v can be described as a wave with wavelength lambda = Planck’s constant divided by (m × v).
2. Schrödinger Equation (1926): The time-independent form for a single electron in a central electric potential V(r)...
3. Spin Quantum Number: Discovered by Goudsmit and Uhlenbeck in 1925...
4. Atomic Orbitals and Probability Densities: Each allowed set of quantum numbers (n, ℓ, m_ℓ) defines an orbital with a characteristic shape and energy.
Key Insight: The quantum mechanical model shows that electrons are not tiny planets circling the nucleus; instead, each electron occupies an orbital—a region in which there is a certain probability of finding it.
Detailed Explanation
The quantum mechanical model revolutionized atomic theory by incorporating wave-particle duality. This model indicates that particles such as electrons exhibit both wave-like and particle-like properties. The behavior of electrons is described by a wave function, indicating that they exist in orbitals, which are regions of probability rather than fixed paths. The concept of quantum numbers helps define the specific characteristics of these orbitals, including their shape, orientation, and energy.
Examples & Analogies
Imagine throwing a handful of winter snowflakes into the air—while you can predict the general area where they will fall, you cannot know their exact position at any moment. Similarly, in the quantum model, we can predict where an electron is likely to be around a nucleus, but we cannot pinpoint its exact location at all times.
Electron Energy Levels and Orbitals
Chapter 3 of 6
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
2.2 Principal Energy Levels, Sublevels, and Orbitals
In a multielectron atom, electrons occupy energy levels (shells) and sublevels (subshells), filling from lowest energy upward. Each electron is uniquely described by the four quantum numbers (n, ℓ, m_ℓ, m_s).
2.2.1 Principal Quantum Number (n): Indicates the main energy level or “shell” of the electron, roughly correlating with the average distance of the electron from the nucleus.
2.2.2 Azimuthal (Angular Momentum) Quantum Number (ℓ): Defines the subshell and orbital shape.
2.2.3 Magnetic Quantum Number (m_ℓ): Specifies the orientation of the orbital in space.
2.2.4 Spin Quantum Number (m_s): Specifies the direction of the electron’s intrinsic spin.
Detailed Explanation
Electrons are arranged in specific energy levels or shells around the nucleus. The principal quantum number (n) indicates these levels, starting from 1 for the closest to the nucleus. Each energy level can contain sublevels defined by the azimuthal quantum number (ℓ), which determine the shape of the orbitals (s, p, d, f). Finally, the magnetic quantum number (m_ℓ) describes the orientation of these orbitals, and the spin quantum number (m_s) indicates the spin direction of the electron within each orbital.
Examples & Analogies
Consider a hotel with multiple floors (energy levels). Each floor has different types of rooms (sublevels: suites, doubles, singles). The individual rooms (orbitals) can either be occupied by one or two guests (electrons), where the arrangement of guests varies depending on the room size and orientation.
Electron Configuration Principles
Chapter 4 of 6
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
2.3 Aufbau Principle, Pauli Exclusion Principle, and Hund’s Rule
When building the ground-state (lowest-energy) electron configuration of an atom:
1. Aufbau Principle (“Building Up”): Electrons occupy the lowest-energy orbitals available before filling higher-energy ones.
2. Pauli Exclusion Principle: Each orbital can hold at most two electrons, and those two must have opposite spins.
3. Hund’s Rule of Maximum Multiplicity: When electrons occupy a set of degenerate orbitals (orbitals of exactly the same energy), they fill each orbital singly first, all with parallel spins...
Detailed Explanation
These principles guide how electrons are distributed among the available orbitals in an atom. According to the Aufbau principle, electrons fill the lowest available energy levels first, ensuring stability. The Pauli Exclusion Principle states that no two electrons can have the same set of quantum numbers, meaning that each orbital can hold a maximum of two electrons with opposite spins. Hund’s Rule clarifies that when filling orbitals of the same energy, electrons prefer to occupy separate orbitals first, which minimizes repulsion.
Examples & Analogies
Think of a team choosing seats in a movie theater. They will first fill the available seats in the front row (lowest energy levels) before moving towards the back. If multiple seats in the same row are open, they will each pick an unoccupied seat before sitting together to maximize their comfort and space.
Writing Electron Configurations
Chapter 5 of 6
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
2.4 Writing Electron Configurations
2.4.1 Standard Notation: ...
2.4.2 Noble Gas Core Notation: ...
Detailed Explanation
Writing electron configurations involves expressing how electrons are arranged in an atom’s orbitals. Using standard notation involves listing orbitals in order of increasing energy with superscripts indicating how many electrons occupy each. Noble gas core notation presents a shortcut by using the configuration of the previous noble gas in brackets and then detailing only the additional orbitals, making for a more concise representation.
Examples & Analogies
Imagine filling a library with books. Instead of listing each and every book, you can note the sections (like the noble gas) and indicate how many additional titles you're adding. This way, you streamline the process while keeping everything organized.
Energy Diagrams and Orbital Filling Order
Chapter 6 of 6
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
2.5 Energy Level Diagrams and Orbital Filling Order
2.5.1 Aufbau Order Diagram: ...
2.5.2 Relative Energies of Orbitals: ...
Detailed Explanation
Energy level diagrams visually represent the order in which orbitals fill based on energy levels. By drawing arrows, one can illustrate electron placement, respecting the rules set by the Aufbau principle, Pauli Exclusion Principle, and Hund’s rule. Understanding how these orbitals relate in terms of energy—where s-orbitals are filled before p, d, or f—helps students grasp not only the structure of elements but also predict their behavior in reactions.
Examples & Analogies
Think of a pyramid, where the lowest layer can hold the most people (s-orbitals), while the higher layers (p, d, f) become progressively more exclusive. Participants must fill up the base first before moving on to the next higher tier, ensuring that everyone has an equal opportunity to gather space.
Key Concepts
-
Electron Configuration: The arrangement of electrons in atom's orbitals determined by quantum mechanics principles.
-
Aufbau Principle: Governs the order of filling of electron orbitals.
-
Pauli Exclusion Principle: No two electrons can have the same four quantum numbers.
-
Hund's Rule: Electrons fill degenerate orbitals singly before pairing to minimize repulsion.
-
Quantum Numbers: Four values (n, ℓ, m_ℓ, m_s) that uniquely describe an electron's state.
Examples & Applications
For carbon, the electron configuration is 1s² 2s² 2p², using standard notation.
Using noble gas core notation, chlorine can be represented as [Ne] 3s² 3p⁵.
Memory Aids
Interactive tools to help you remember key concepts
Rhymes
When filling electrons, low goes first, Aufbau helps us avoid the burst!
Stories
Imagine a dance where every dancer needs a unique outfit. If two dancers wear the same outfit, they can't join the dance floor! This represents the Pauli Exclusion Principle.
Memory Tools
Rule of Three: Aufbau (lowest first), Pauli (no twins allowed), Hund (one at a time).
Acronyms
A P H
Remember the sequence as 'A' for Aufbau
'P' for Pauli
'H' for Hund.
Flash Cards
Glossary
- Electron Configuration
The distribution of electrons in an atom's orbitals, according to energy levels and sublevels.
- Aufbau Principle
A rule stating that electrons occupy the lowest energy orbitals available before filling higher ones.
- Pauli Exclusion Principle
A principle asserting that no two electrons in an atom can have the same set of four quantum numbers.
- Hund's Rule
A rule stating that electrons will fill degenerate orbitals singly with parallel spins before pairing up.
- Quantum Numbers
Numbers that describe the quantized state of an electron, including its energy level, shape, orientation, and spin.
- Orbital
A region in space where there is a high probability of finding an electron, characterized by its shape.
- Noble Gas Core Notation
A shorthand method of writing electron configurations that uses the electron configuration of the nearest noble gas in brackets.
Reference links
Supplementary resources to enhance your learning experience.
