Functional Groups
Enroll to start learning
You’ve not yet enrolled in this course. Please enroll for free to listen to audio lessons, classroom podcasts and take practice test.
Interactive Audio Lesson
Listen to a student-teacher conversation explaining the topic in a relatable way.
Introduction to Functional Groups
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Today, we will discuss functional groups, which are essential in determining the chemical properties of organic compounds. Can anyone tell me what they think a functional group is?

Is it a specific atom or group of atoms that gives a compound its properties?

Exactly! Functional groups are part of the molecules that largely dictate their reactivity. Let’s explore some examples.
Alcohols
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

One important functional group is the alcohol group, represented as -OH. This is called the hydroxyl group. Who can tell me an example of an alcohol?

Isn't ethanol an alcohol?

Correct! Ethanol has the formula C₂H₅OH. Alcohols can hydrogen bond, affecting their physical properties. Now, what can you tell me about their reactivity?

They can undergo oxidation to become acids.

That's right! Understanding this is crucial for organic synthesis. Remember: Alcohol = Hydroxyl Group = Reactivity Modifier.
Carboxylic Acids
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Next, let’s discuss carboxylic acids, which contain the -COOH group. What do you think are some characteristics of carboxylic acids?

I remember they are weak acids and can donate protons!

Exactly! An example of this is ethanoic acid. Their acidic nature comes from the carboxyl group. Now, can anyone think of a reaction where carboxylic acids play a role?

They can react to form esters!

Well said! So, remember: Carboxylic Acid = Acidic Nature = Reactivity Modifier.
Halides
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Our last functional group for today is halides, which can be -Cl, -Br, etc. What can you tell me about them?

I think they are important in synthesis and can have different properties based on the halogen.

Yes, halides can act as intermediates in organic reactions! For instance, methyl chloride can participate in substitution reactions. So, remember the key: Halides = Reactivity Intermediates.
Introduction & Overview
Read summaries of the section's main ideas at different levels of detail.
Quick Overview
Standard
Each functional group (such as alcohols, carboxylic acids, and halides) is defined by its unique atom or arrangement of atoms, influencing how the compound behaves chemically. Understanding functional groups is crucial in organic chemistry as they are key indicators of reactivity and properties.
Detailed
Detailed Summary
Functional groups are crucial in organic chemistry as they define the chemical behavior of carbon-based compounds. A functional group is an atom or a group of atoms within a molecule that is primarily responsible for the characteristic chemical reactions of that molecule. In this section, three significant functional groups are discussed:
- Alcohols (-OH): Characterized by the hydroxyl group, alcohols can participate in hydrogen bonding, influencing their boiling points and solubility.
- Example: Ethanol (C₂H₅OH)
- Carboxylic Acids (-COOH): These contain the carboxyl group and are weak acids, often found in organic reactions due to their acidic nature.
- Example: Ethanoic acid (CH₃COOH)
- Halides (-Cl, -Br): Compounds with halogen atoms which can serve as important intermediates in chemical synthesis.
- Examples: Methyl chloride (CH₃Cl), Ethyl bromide (C₂H₅Br)
Understanding functional groups enables chemists to predict the reactivity and interactions of organic compounds, forming the basis for further study of organic reactions and mechanisms.
Youtube Videos








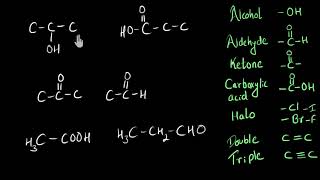

Audio Book
Dive deep into the subject with an immersive audiobook experience.
Definition of Functional Group
Chapter 1 of 2
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
● A functional group is an atom or group of atoms that determines the chemical properties of a compound.
Detailed Explanation
A functional group is essentially the part of a molecule that is responsible for its chemical reactions. Each functional group has specific properties that dictate how the compound will behave in chemical reactions, allowing chemists to predict the behavior of organic compounds based on their functional groups.
Examples & Analogies
Think of a functional group like a car model's features (e.g., sunroof, leather seats) that define how the car performs. Just as the features determine how comfortable and enjoyable a car ride will be, the functional group determines how a compound will react with other substances.
Examples of Functional Groups
Chapter 2 of 2
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
| Functional Group | Formula | Example |
|---|---|---|
| Alcohol | –OH | Ethanol (C₂H₅OH) |
| Carboxylic Acid | –COOH | Ethanoic acid (CH₃COOH) |
| Halides | –Cl, –Br | CH₃Cl, C₂H₅Br |
Detailed Explanation
Several common functional groups include:
- Alcohols (–OH): Compounds containing a hydroxyl group that are often polar and can engage in hydrogen bonding, impacting their solubility and boiling points.
- Carboxylic Acids (–COOH): Characterized by a carbonyl and a hydroxyl group, they are acidic and found in vinegar (ethanoic acid).
- Halides (–Cl, –Br): Organic compounds that include halogen atoms, which can affect reactivity and physical properties.
Examples & Analogies
Imagine different tools in a toolbox. Each tool (functional group) has a specific job (chemical property). For instance, the alcohol group is like a screwdriver—useful for fixing things, just as alcohol can modify the properties of a compound and how it interacts with other substances.
Key Concepts
-
Functional Group: Determines the chemical properties of organic compounds.
-
Alcohols: Contain the -OH group; important for hydrogen bonding.
-
Carboxylic Acids: Contain the -COOH group; weak acids, act as proton donors.
-
Halides: Organic compounds with halogens; useful in synthesis.
Examples & Applications
Ethanol (C₂H₅OH) is an example of an alcohol.
Ethanoic acid (CH₃COOH) is an example of a carboxylic acid.
Methyl chloride (CH₃Cl) is an example of a halide.
Memory Aids
Interactive tools to help you remember key concepts
Rhymes
In the world of organic, make a note, / Alcohols say 'I bond with hope!' / Acids give a proton, that's their call, / Halides make reactions enthrall.
Stories
Imagine a party where everyone has a character: Alcohols bring the laughter (hydroxyl group), carboxylic acids are the life of the party with their fiery nature (acidity), and halides act as covert agents, helping facilitate all the exits (reactions).
Memory Tools
Remember: A Group of Friends - Alcohols (A) for hugs, Acids (B) for fire, and Halides (C) for secrets and reactions.
Acronyms
AHA - Alcohols, Halides, Acids - remember these three main functional groups!
Flash Cards
Glossary
- Functional Group
An atom or group of atoms responsible for the characteristic chemical reactions of a compound.
- Alcohol
An organic compound containing a hydroxyl (-OH) group.
- Carboxylic Acid
An organic acid containing a carboxyl (-COOH) group.
- Halide
An organic compound containing one or more halogen atoms (such as Cl, Br).
Reference links
Supplementary resources to enhance your learning experience.
