Saturated Hydrocarbons (Alkanes)
Enroll to start learning
You’ve not yet enrolled in this course. Please enroll for free to listen to audio lessons, classroom podcasts and take practice test.
Interactive Audio Lesson
Listen to a student-teacher conversation explaining the topic in a relatable way.
Introduction to Saturated Hydrocarbons
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Today, we're going to learn about saturated hydrocarbons, also called alkanes. Can anyone tell me what defines a saturated hydrocarbon?

Are they the ones that only have single bonds?

Exactly! Alkanes consist solely of carbon and hydrogen with all single bonds. They are represented by the general formula CnH2n+2. This means for every carbon atom, there are usually twice as many hydrogen atoms plus two more!

So, what would be an example of an alkane?

Great question, Student_2! Methane (CH₄) and ethane (C₂H₆) are both examples of alkanes. Can anyone tell me the number of hydrogen atoms in ethane?

Should be six, right?

Correct! And you'll notice that each alkane increases by a –CH₂– group, making it part of a homologous series.
The General Formula of Alkanes
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Now, let’s break down the general formula for alkanes: CnH2n+2. What happens if we have one carbon atom, n = 1?

That would be CH₄, methane!

Yes! So if n is increased to 2, what would the formula provide us?

That’d be C₂H₆, which is ethane!

Exactly! Following the same pattern, if we have three carbon atoms, what would we get?

C₃H₈, which is propane!

Correct! This systematic way of identifying alkanes makes understanding their properties much easier.
Significance and Uses of Alkanes
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Now that we understand what alkanes are, can anyone tell me why they are important in the field of organic chemistry?

Maybe because they form the base for other compounds?

Exactly! They serve as foundational compounds. Alkanes can be used as fuels, like methane in natural gas or propane in gas grills.

Are they reactive?

Generally, they are less reactive compared to their unsaturated counterparts. This stability is what makes them useful in various applications. Can anyone think of other applications for alkanes?

I guess they could be used in the production of plastics?

Precisely! Alkanes are also used to synthesize many organic compounds, showcasing their versatility.
Introduction & Overview
Read summaries of the section's main ideas at different levels of detail.
Quick Overview
Standard
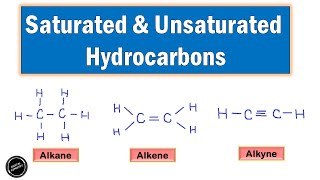
Saturated hydrocarbons, also known as alkanes, are characterized by their single bonds between carbon atoms and follow the general formula CnH2n+2. Common examples include methane (CH₄) and ethane (C₂H₆). These compounds play a fundamental role in organic chemistry due to their stability and simplicity.
Detailed
Saturated Hydrocarbons (Alkanes)
Saturated hydrocarbons, commonly referred to as alkanes, are a subclass of hydrocarbons consisting of only carbon and hydrogen atoms connected exclusively by single covalent bonds. They follow the general formula CₙH₂ₙ₊₂, where n represents the number of carbon atoms in the molecule.
Examples:
- Methane (CH₄): The simplest alkane consisting of one carbon atom and four hydrogen atoms.
- Ethane (C₂H₆): Comprising two carbon atoms and six hydrogen atoms.
Saturated hydrocarbons are significant in organic chemistry due to their widespread occurrence, relative stability, and the foundational role they play in forming other organic compounds.
Youtube Videos








Audio Book
Dive deep into the subject with an immersive audiobook experience.
Definition of Saturated Hydrocarbons
Chapter 1 of 3
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Saturated hydrocarbons, also known as alkanes, are characterized by having all single bonds between carbon atoms.
Detailed Explanation
Saturated hydrocarbons are a type of hydrocarbon where all the bonds connecting carbon atoms are single bonds. This means that each carbon atom is bonded to other carbon atoms or hydrogen atoms without any double or triple bonds. The single bonds allow for the maximum number of hydrogen atoms to be attached to the carbon chain, making these molecules 'saturated' with hydrogen.
Examples & Analogies
Think of saturated hydrocarbons like a string of pearls, where each pearl represents a carbon atom. If all the pearls are connected with single loops of string (single bonds) without any twists (double or triple bonds), then the string is fully 'saturated' without any gaps.
General Formula for Alkanes
Chapter 2 of 3
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
The general formula for saturated hydrocarbons (alkanes) is CₙH₂ₙ₊₂.
Detailed Explanation
This formula represents the relationship between the number of carbon atoms (Cₙ) and the number of hydrogen atoms (H₂ₙ₊₂) in an alkane. For every carbon atom in the molecule, there are two hydrogen atoms plus an additional two hydrogen atoms to account for the ends of the carbon chain. This formula is useful for predicting the number of hydrogen atoms in a given alkane as you increase the number of carbon atoms.
Examples & Analogies
Imagine you are building a chain with different numbers of ‘pearls’. If you start with 1 carbon pearl, you can attach 4 hydrogen pearls, and if you add another carbon pearl to the chain, you can add 6 hydrogen pearls, and so on. This shows how the formula builds as you add more carbon atoms.
Examples of Alkanes
Chapter 3 of 3
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Examples include methane (CH₄) and ethane (C₂H₆).
Detailed Explanation
Methane is the simplest alkane, consisting of one carbon atom and four hydrogen atoms (CH₄). Ethane follows, with two carbon atoms and six hydrogen atoms (C₂H₆). These examples highlight how alkanes start from just one carbon and build upwards, showcasing their structural simplicity and commonality.
Examples & Analogies
Think of these alkanes as basic recipes for making simple dishes. Just as you can make a one-ingredient dish (methane) or a dish with double the ingredients (ethane), alkanes can be seen as building blocks that can be extended in variety while still maintaining a simple structure.
Key Concepts
-
Saturated Hydrocarbons: Compounds consisting of carbon and hydrogen only, with all single bonds.
-
General Formula: Alkanes follow the formula CnH2n+2 where n is the number of carbon atoms.
-
Examples of Alkanes: Methane (CH₄), Ethane (C₂H₆), and Propane (C₃H₈).
Examples & Applications
Methane (CH₄): The simplest alkane consisting of one carbon atom and four hydrogen atoms.
Ethane (C₂H₆): Comprising two carbon atoms and six hydrogen atoms.
Saturated hydrocarbons are significant in organic chemistry due to their widespread occurrence, relative stability, and the foundational role they play in forming other organic compounds.
Memory Aids
Interactive tools to help you remember key concepts
Rhymes
Alkanes are stable, with hydrogen so bold, single bonds they hold, in structures unfold.
Stories
Once in a lab, a young chemist named Al introduced alkanes who were gathered round. With one bond a piece and hydrogen galore, they made stable friends and chemistry soared!
Memory Tools
To remember alkanes, think 'A Does Not Like Double Bonds' – A for Alkanes, D for Double bonds!
Acronyms
S.H.E. (Saturated Hydrocarbons with Exclusive single bonds) can help recall the main attributes of alkanes.
Flash Cards
Glossary
- Alkanes
A class of hydrocarbons that contain only single bonds between carbon atoms, following the formula CnH2n+2.
- Saturated Hydrocarbons
Hydrocarbons lacking double or triple bonds; all carbon atoms are saturated with hydrogen.
- Homologous Series
A series of compounds with the same functional group and similar chemical properties, differing by a –CH₂– group.
- Methane
The simplest alkane, with the chemical formula CH₄.
Reference links
Supplementary resources to enhance your learning experience.
