Introduction to p-Block Elements
Enroll to start learning
You’ve not yet enrolled in this course. Please enroll for free to listen to audio lessons, classroom podcasts and take practice test.
Interactive Audio Lesson
Listen to a student-teacher conversation explaining the topic in a relatable way.
Overview of p-Block Elements
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Let's begin today by discussing p-Block elements, which are located in Groups 13 to 18 of the periodic table. Does anyone know why they're called 'p-block' elements?

Because their outermost electrons go into the p-orbital?

Exactly right, Student_1! The p-orbital is where the outer electrons are located. This includes metals, non-metals, and even metalloids. Can anyone name some common p-block elements?

I think Carbon, Nitrogen, and Oxygen are examples.

And Chlorine and Sulfur too!

Great! These elements are essential for life. For instance, Carbon is fundamental to all organic chemistry. Remember the acronym CNO—Carbon, Nitrogen, and Oxygen. They are key elements!

Why are they so important for living things?

These elements are crucial because they form the building blocks of life. For example, Oxygen is vital for respiration. To summarize, p-Block elements play significant roles in many biological and industrial processes!
Types of p-Block Elements
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Now that we know what p-Block elements are, let’s explore the different types within this group: metals, non-metals, and metalloids. What do you think differentiates these types?

Metals are usually shiny and conductive, right?

Correct! Metals are good conductors of electricity and heat. Non-metals, on the other hand, often lack lustre and are insulators. Who can give me an example of a metalloid?

Silicon is a metalloid, isn’t it?

Yes! Silicon can conduct electricity but not as well as metals. Remember the mnemonic: 'MNM' for Metals, Non-metals, and Metalloids. Each type contributes differently, influencing their reactions and applications.

What about their oxidation states?

Excellent question! p-Block elements can exhibit variable oxidation states, which means they can lose or gain different numbers of electrons. This variability is crucial for their chemical behavior.

So they can react in different ways?

Exactly! This versatility underpins many chemical reactions in nature. To conclude, the p-Block elements' diversity allows them to play various roles in both biological and industrial contexts.
Importance of p-Block Elements
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Let's shift our focus to the importance of p-Block elements. Can anyone tell me why we study them in our daily lives?

They are part of many things we use like water and air!

Correct! Elements like Oxygen and Nitrogen are found in the air. Can anyone identify some compounds that contain p-Block elements?

Water (H₂O) is one, and table salt (NaCl) too!

Exactly! Water is vital for life, and sodium chloride is crucial for flavor and preservation. I want you to remember the phrase 'Oxygen keeps us alive'—it highlights its importance in respiration.

I heard that some p-Block elements can also cause pollution. Is that true?

Yes! For example, Nitrogen oxides can contribute to acid rain. It's really important to understand both their benefits and potential harms. To sum up, p-Block elements are not only part of our daily lives but also play important roles in numerous chemical processes and reactions!
Introduction & Overview
Read summaries of the section's main ideas at different levels of detail.
Quick Overview
Standard
This section introduces p-Block elements, which include metals, non-metals, and metalloids found in Groups 13 to 18 of the periodic table. The focus is on known elements like Carbon, Nitrogen, Oxygen, Sulfur, and Chlorine, highlighting their roles in life and industry.
Detailed
Introduction to p-Block Elements
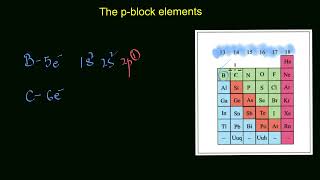
The p-Block elements are situated in Groups 13 to 18 of the periodic table. The name 'p-block' comes from the fact that these elements have their outermost electrons entering the p-orbital. They include a mix of metals, non-metals, and metalloids, each exhibiting a variety of properties and oxidation states. In Class 8, you will focus on pivotal p-block elements such as Carbon (C), Nitrogen (N), Oxygen (O), Sulfur (S), and Chlorine (Cl). These elements play crucial roles in biological systems and are vital for various industrial applications. Understanding p-block elements is essential as they form the backbone of organic compounds, aid in respiration, and are involved in many environmental processes.
Youtube Videos




Audio Book
Dive deep into the subject with an immersive audiobook experience.
Definition and Location of p-Block Elements
Chapter 1 of 4
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
● p-Block elements are found in Groups 13 to 18 of the periodic table.
Detailed Explanation
p-Block elements occupy positions in the periodic table from Group 13 to Group 18. This range includes a variety of elements, each with distinct properties. Understanding where these elements are located helps us relate their properties and behaviors to their position in the table.
Examples & Analogies
Think of the periodic table as a family tree, where each branch represents a group of elements. The p-block is like a section of this family tree that houses diverse relatives—some are like metals (like aluminum), while others act like non-metals (like oxygen).
Nature of Outer Electrons
Chapter 2 of 4
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
● They are called p-block because their outermost electrons enter the p-orbital.
Detailed Explanation
The name 'p-block' comes from the electronic configuration of these elements. The outermost electrons of these elements fill the p-orbitals, which are a specific type of atomic orbital. This filling pattern significantly influences their chemical behaviors and bonding capabilities.
Examples & Analogies
Imagine the p-orbitals as rooms in a house where guests (electrons) stay. How many rooms are filled and how many guests are there determines the overall party atmosphere (or chemical behavior). Just as more guests can lead to more interaction and fun, a filled p-orbital leads to diverse chemical reactions.
Types of p-Block Elements
Chapter 3 of 4
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
● Includes metals, non-metals, and metalloids.
Detailed Explanation
The p-block consists of three categories of elements: metals, non-metals, and metalloids. Metals are typically good conductors of electricity and heat, non-metals can be insulators and vary widely in properties, and metalloids have characteristics of both metals and non-metals, making them versatile.
Examples & Analogies
Think of the p-block elements like a sports team comprised of various players with different skills. The metals are the strong players (like defenders), non-metals are the agile and quick players (like forwards), and metalloids are the versatile players who can play multiple positions, adapting to what the team needs.
Common p-Block Elements
Chapter 4 of 4
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
● Common p-block elements in Class 8 focus: Carbon, Nitrogen, Oxygen, Sulfur, and Chlorine.
Detailed Explanation
In studies, students often focus on key p-block elements such as Carbon (C), which is fundamental in organic chemistry, Nitrogen (N), essential for fertilizers, Oxygen (O), crucial for life, Sulfur (S), known for its role in compounds like sulfuric acid, and Chlorine (Cl), important for disinfection. Understanding these elements gives a strong foundation in chemistry.
Examples & Analogies
Imagine these common p-block elements as the ingredients in a recipe. Each ingredient adds a unique flavor and function to the final dish. Carbon is like the base flavor, Nitrogen provides nutritional value, Oxygen is essential for perfect cooking, Sulfur adds depth, and Chlorine ensures cleanliness—together they create a balanced and beneficial outcome.
Key Concepts
-
p-Block Elements: Elements in Groups 13 to 18 of the periodic table with outer electrons in p-orbitals.
-
Metals: Shiny, conductive elements found in the p-block.
-
Non-Metals: Poor conductors and non-lustrous elements in the p-block.
-
Metalloids: Elements with mixed properties of metals and non-metals.
-
Oxidation States: Indicate how many electrons can be gained or lost by elements.
Examples & Applications
Carbon is fundamental in organic chemistry and forms various compounds.
Oxygen is essential for respiration in living organisms.
Chlorine is used in disinfection and water purification processes.
Memory Aids
Interactive tools to help you remember key concepts
Rhymes
In p-Block, electrons dance in p, Metals, non-metals, can't you see?
Stories
Once upon a time in Element Town, Metals shined bright, while Non-Metals wore a frown. Metalloids stood in the middle, playing both sides, showcasing diversity like a colorful tide.
Memory Tools
Remember 'MNM' for Metals, Non-metals, and Metalloids in the p-Block.
Acronyms
Use the acronym CNO to remember Carbon, Nitrogen, Oxygen as essential p-block elements.
Flash Cards
Glossary
- pBlock Elements
Elements located in Groups 13 to 18 of the periodic table, characterized by the filling of p-orbitals.
- Metals
Elements that are typically shiny, good conductors of heat and electricity, and malleable.
- NonMetals
Elements that lack metallic properties, often poor conductors and not lustrous.
- Metalloids
Elements that have properties intermediate between metals and non-metals.
- Oxidation States
The degree of oxidation of an element, indicating how many electrons it can lose or gain.
Reference links
Supplementary resources to enhance your learning experience.
