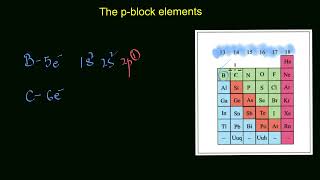
Oxygen (O) – Group 16
Enroll to start learning
You’ve not yet enrolled in this course. Please enroll for free to listen to audio lessons, classroom podcasts and take practice test.
Interactive Audio Lesson
Listen to a student-teacher conversation explaining the topic in a relatable way.
Introduction to Oxygen
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Today, we will discuss oxygen, which is an essential element in Group 16 of the periodic table. Can anyone tell me what percentage of the atmosphere oxygen makes up?

Is it 21%?

Exactly! Oxygen makes up about 21% of the atmosphere. It’s crucial for respiration. What do we mean by respiration?

It’s how animals and plants take in oxygen and release carbon dioxide!

Great answer! So, oxygen is vital for life on Earth. Remember the acronym 'O2 R' – where 'O2' stands for oxygen in its diatomic form and 'R' for respiration.
Oxygen and Combustion
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Now, let’s talk about combustion. Can anyone explain how oxygen plays a role here?

Oxygen supports burning. Without it, fire cannot exist.

Correct! Think of oxygen as the fuel for fire. You can remember it using the mnemonic 'Oxygen Fuels Fire.' What happens if there is no oxygen during combustion?

The fire will go out!

Exactly! So, how does oxygen form compounds with other elements?
Oxygen and Oxides
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Oxygen is known to form oxides with almost every element. What can you tell me about oxides?

Oxides are compound molecules consisting of oxygen and another element.

That's correct! Can anyone name a few examples of oxides?

I think carbon dioxide (CO2) and water (H2O) are oxides.

Yes! Water (H₂O) is an oxide of hydrogen, and carbon dioxide (CO₂) is an oxide of carbon. Remember, 'Oxides are everywhere!'

Is ozone also related to oxygen?

Yes, good question! Ozone (O₃) is an allotrope of oxygen. It plays a critical role in protecting Earth from UV radiation.
Ozone and Its Importance
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Let’s discuss ozone. Who remembers what this allotrope of oxygen does for us?

Ozone protects us from harmful UV rays from the sun!

Exactly! Ozone forms a layer in the stratosphere. It’s crucial for keeping us safe. Can anyone think of why too much UV exposure can be harmful?

It can cause skin cancer and harm our eyes!

Great points! We can remember ozone's protective role with the phrase 'Ozone Saves Life.'
Introduction & Overview
Read summaries of the section's main ideas at different levels of detail.
Quick Overview
Standard
Oxygen, a key member of Group 16 in the periodic table, plays a crucial role in respiration and combustion processes. It forms oxides with nearly all elements and has an allotrope known as ozone that protects against UV radiation.
Detailed
Detailed Summary of Oxygen (O)
Oxygen (O), which is part of Group 16 in the periodic table, makes up about 21% of the Earth's atmosphere. It is essential for both respiration in living organisms and combustion processes vital for energy release. Notably, oxygen supports burning but does not combust on its own. It readily combines with almost all elements to form various oxides, making it an incredibly reactive element. Another important form of oxygen is ozone (O₃), an allotrope that forms a protective layer in the stratosphere, absorbing harmful ultraviolet (UV) radiation from the sun. The properties and significance of oxygen underscore its essential role in both biological systems and environmental processes.
Youtube Videos




Audio Book
Dive deep into the subject with an immersive audiobook experience.
Oxygen Composition in the Atmosphere
Chapter 1 of 5
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
● Makes up 21% of the atmosphere.
Detailed Explanation
Oxygen is a major component of the Earth's atmosphere, constituting about 21% of the air we breathe. This percentage is significant because it enables life on Earth to exist, as many organisms, including humans, require oxygen for survival.
Examples & Analogies
Think of the oxygen in the atmosphere as the fuel that keeps our bodies running, just like gasoline is essential for cars to operate. Without gasoline, cars cannot run; similarly, without oxygen, we cannot breathe or live.
Essential Functions of Oxygen
Chapter 2 of 5
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
● Essential for respiration and combustion.
Detailed Explanation
Oxygen plays a crucial role in two vital processes: respiration and combustion. In respiration, organisms like humans inhale oxygen, which is then used to convert food into energy. In combustion, oxygen is needed to ignite and sustain fires, allowing for heating, cooking, and various industrial processes.
Examples & Analogies
You can think of respiration like a factory that transforms raw materials (food) into energy (electricity) using oxygen as a key ingredient for the process. Just as factories need fuel to operate, living beings require oxygen for their energy production.
Oxygen's Role in Combustion
Chapter 3 of 5
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
● Supports burning but does not burn itself.
Detailed Explanation
While oxygen is essential for combustion, it itself does not burn. Instead, it supports the burning of other materials by reacting with them. This process generates heat and light, which we observe when something catches fire.
Examples & Analogies
Imagine oxygen as a person holding a matchstick (representing fuel). The matchstick needs the oxygen to ignite and burn, but the person cannot burn. They facilitate the fire but are not part of it, just like oxygen supports combustion without burning itself.
Formation of Oxides
Chapter 4 of 5
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
● Forms oxides with almost all elements.
Detailed Explanation
Oxygen readily reacts with many elements to form compounds known as oxides. These compounds are essential in many chemical reactions and are crucial in various industries and biological processes. For instance, when oxygen combines with hydrogen, it forms water (H₂O), a vital substance for life.
Examples & Analogies
Think of oxygen as a team player in a sport. It collaborates with different elements, forming strong partnerships (oxides) that lead to important outcomes, such as water, which is essential for hydration and agriculture.
Ozone: A Unique Allotrope of Oxygen
Chapter 5 of 5
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
● Ozone (O₃) is an allotrope of oxygen; protects Earth from UV radiation.
Detailed Explanation
Ozone is a special form of oxygen composed of three oxygen atoms (O₃), rather than the usual two (O₂). Ozone forms a layer in the Earth's stratosphere, which absorbs and protects us from harmful ultraviolet (UV) radiation from the sun. Without this ozone layer, life on Earth would be severely affected as UV rays can cause skin damage and other health problems.
Examples & Analogies
Imagine the ozone layer as a sunscreen for our planet. Just as sunscreen protects our skin from the sun's harmful rays, the ozone layer shields Earth from UV radiation, helping keep both humans and ecosystems safe.
Key Concepts
-
Oxygen's role in respiration: Essential for living organisms as it is required for breathing.
-
Oxygen in combustion: Supports burning but does not burn itself.
-
Formation of oxides: Oxygen reacts with almost all elements to form oxides.
-
Role of ozone: Protects the Earth from harmful UV radiation.
Examples & Applications
The air we breathe is composed mainly of oxygen and nitrogen.
Water (H₂O) and carbon dioxide (CO₂) are common oxides formed by oxygen.
Memory Aids
Interactive tools to help you remember key concepts
Rhymes
Oxygen’s everywhere, it fills the air, supports our life, and makes fire flare.
Stories
Once upon a time, in a world without oxygen, fire couldn't exist, and living creatures struggled to survive. They dreamt of the day when oxygen would fill their realm, allowing them to breathe, thrive, and even light their fires.
Memory Tools
Remember 'R.O.C.' - Respiration, Oxidation, Combustion to remember oxygen’s roles.
Acronyms
Use 'O2 G.P.' - Oxygen 2 Go to symbolize its essential functions in life
Gathering supplies for respiration.
Flash Cards
Glossary
- Oxygen
A diatomic molecule essential for respiration, making up 21% of the Earth's atmosphere.
- Combustion
The process of burning that requires oxygen to release energy.
- Oxide
A compound formed when oxygen combines with another element.
- Ozone
An allotrope of oxygen (O₃) that protects against UV radiation.
Reference links
Supplementary resources to enhance your learning experience.
