Some p-Block Elements
Enroll to start learning
You’ve not yet enrolled in this course. Please enroll for free to listen to audio lessons, classroom podcasts and take practice test.
Interactive Audio Lesson
Listen to a student-teacher conversation explaining the topic in a relatable way.
Introduction to p-Block Elements
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Today, we will explore p-block elements! Can anyone tell me what p-block elements are?

Are they the elements in groups 13 to 18 of the periodic table?

Excellent! Yes, these elements are found in groups 13 to 18, and their electrons fill the p-orbital. Can anyone name a few examples?

Carbon, nitrogen, oxygen…

And sulfur and chlorine!

Great job! These include metals, non-metals, and metalloids. To remember the elements we usually study, you can use the acronym 'C-N-O-S-Cl.'

That sounds useful!

Let's summarize: p-block elements are groups 13-18, their outermost electrons are in the p-orbital, and they include both metals and non-metals. Who can summarize what we've discussed?

They fill p-orbitals, are found in groups 13-18, and include different types of elements!
Characteristics of p-Block Elements
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Now, let's talk about the general characteristics of p-block elements. Can anyone think of what makes them special?

They can have different oxidation states?

Absolutely! They show variable oxidation states. This is important because it allows them to participate in various chemical reactions. What else?

Some are essential for life!

That's right! Elements like oxygen, carbon, and nitrogen are crucial for living organisms. Can anyone provide an example of where we find these elements?

In the air, water, and even in our bodies!

Fantastic! p-block elements are pervasive in our environment and are essential for many compounds and processes. Let’s summarize: they have variable oxidation states and many are vital for life.
Carbon and Its Compounds
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Let’s focus on carbon now. What can you tell me about its forms?

It has crystalline forms like diamond and graphite!

Correct! And it also has amorphous forms like charcoal and coal. Why do you think carbon is so important in chemistry?

Because it can form so many compounds!

Exactly! Organic chemistry is based on carbon compounds. Can anyone mention two important oxides of carbon?

Carbon dioxide and carbon monoxide!

Great! So, carbon dioxide is a product of respiration. Let's summarize: carbon is versatile, exists in various forms, and is essential for a vast array of compounds.
Nitrogen and Its Uses
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Next, we will discuss nitrogen. Who can tell me something interesting about nitrogen?

It makes up 78% of the Earth's atmosphere!

Spot on! Nitrogen is colorless and odorless. What are some uses of nitrogen?

It's used in fertilizers and to make ammonia!

Exactly! The Haber process is key in converting nitrogen to ammonia. What about its role in pollution?

Nitrogen oxides can cause acid rain!

Correct! Let's recap: nitrogen is abundant, essential for ammonia production, and can contribute to environmental issues.
Introduction & Overview
Read summaries of the section's main ideas at different levels of detail.
Quick Overview
Standard
The p-block elements include metals, non-metals, and metalloids from groups 13 to 18 of the periodic table, showing variable oxidation states and playing critical roles in everyday life. Carbon, nitrogen, oxygen, sulfur, and chlorine are the key elements explored, emphasizing their properties and applications.
Detailed
Some p-Block Elements
Introduction
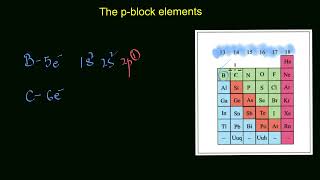
The p-block elements are a diverse group of elements found in groups 13 to 18 of the periodic table. Their name originates from the fact that their outermost electrons are found in the p-orbital. This group includes a variety of metals, non-metals, and metalloids. In this section, we focus on common p-block elements that are significant at the Class 8 level: carbon (C), nitrogen (N), oxygen (O), sulfur (S), and chlorine (Cl).
General Characteristics
p-Block elements exhibit variable oxidation states and can display both metallic and non-metallic properties. Many of these elements are essential to life; for instance, oxygen, nitrogen, and carbon are integral to biological processes. Their compounds are prevalent in the environment, including the air we breathe, the water we drink, and the Earth's crust.
Individual Elements
1. Carbon (C) - Group 14
A versatile non-metal, carbon exists in forms such as crystalline (diamond, graphite) and amorphous (charcoal, coal). It is the cornerstone of organic chemistry as it can form a vast number of compounds. Significant oxides include carbon dioxide (CO₂), which aids in respiration, and carbon monoxide (CO), a hazardous gas.
2. Nitrogen (N) - Group 15
Nitrogen is a colorless and odorless gas making up 78% of the atmosphere. It is largely inert and plays crucial roles in manufacturing ammonia (NH₃) as well as food preservation and fertilization. Its oxides are significant contributors to acid rain and air pollution.
3. Oxygen (O) - Group 16
Oxygen comprises 21% of the atmosphere and is vital for respiration and combustion processes. While it supports combustion, it does not burn itself. Notably, ozone (O₃), an allotrope of oxygen, provides a protective layer against harmful UV radiation.
4. Sulfur (S) - Group 16
Sulfur is a yellow, brittle non-metal commonly found in volcanic regions. It forms compounds such as sulfur dioxide (SO₂), which is used as a preservative but contributes to acid rain, and sulfuric acid (H₂SO₄), crucial in various industries. It has applications in rubber vulcanization and medicines.
5. Chlorine (Cl) - Group 17 (Halogens)
Chlorine is a greenish-yellow gas known for its strong odor and reactivity. It finds extensive use in disinfection and bleaching processes. Notable compounds include hydrochloric acid (HCl) and sodium chloride (NaCl), the latter being common table salt. Its applications also extend to water purification and producing household bleach.
Importance of p-Block Elements
These elements are pivotal in biological, industrial, and environmental processes, being essential for life and widely found in everyday products like plastics, fuels, acids, fertilizers, and medicines.
Youtube Videos




Audio Book
Dive deep into the subject with an immersive audiobook experience.
Introduction to p-Block Elements
Chapter 1 of 2
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
p-Block elements are found in Groups 13 to 18 of the periodic table.
They are called p-block because their outermost electrons enter the p-orbital.
Includes metals, non-metals, and metalloids.
Common p-block elements in Class 8 focus: Carbon, Nitrogen, Oxygen, Sulfur, and Chlorine.
Detailed Explanation
p-Block elements refer to a classification of elements in groups 13 to 18 of the periodic table. These elements have their outermost electrons in the p-orbital, which gives rise to a range of diverse chemical properties. This category includes metals, non-metals, and metalloids - each with different characteristics. In Class 8, students typically study five key p-block elements: Carbon, Nitrogen, Oxygen, Sulfur, and Chlorine, which are significant in both nature and various applications.
Examples & Analogies
Think of p-Block elements as different types of musical instruments in an orchestra. Each group of musicians (the groups in the periodic table) plays its role, with some being metals (like trumpets), non-metals (like violins), and metalloids (like cellos) producing various sounds (chemical properties). Together, they create a harmonious performance, much like how these elements interact in nature.
General Characteristics of p-Block Elements
Chapter 2 of 2
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Show variable oxidation states.
Exhibit both metallic and non-metallic properties.
Many are essential to life (e.g., Oxygen, Nitrogen, Carbon).
Their compounds are commonly found in air, water, and the Earth's crust.
Detailed Explanation
p-Block elements exhibit unique characteristics, such as having variable oxidation states. This means they can lose or gain different numbers of electrons, affecting how they combine with other elements. They demonstrate both metallic (like some aluminum properties) and non-metallic properties (like those seen in oxygen). Many of these elements are vital for life; for instance, oxygen, nitrogen, and carbon are fundamental components of organic molecules. Their compounds play critical roles in our environment, being present in air, water, and mineral resources.
Examples & Analogies
Consider a toolbox for a handyman. Just like different tools can perform varying roles (screwdriver, hammer, wrench), p-Block elements can exhibit multiple oxidation states, serving different purposes in chemical reactions. For example, just as a hammer can either build with nails or break things apart, elements like carbon can bond in diverse formations, contributing to both life and industry.
Key Concepts
-
p-Block Elements: Elements in groups 13 to 18 associated with filling p-orbitals.
-
Variable Oxidation States: p-block elements can exhibit multiple oxidation states.
-
Essential Elements: Many p-block elements are crucial for life, such as O₂ and N₂.
-
Carbon Compounds: Carbon is the foundation of organic chemistry and can form a variety of compounds.
-
Role of Nitrogen: Nitrogen is abundant in the atmosphere and essential for fertilizers.
Examples & Applications
Carbon dioxide (CO₂) is released during respiration and used by plants for photosynthesis.
Nitrogen monoxide (NO) contributes to air pollution and can lead to acid rain.
Ozone (O₃) absorbs harmful UV radiation, protecting life on Earth.
Sulfuric acid (H₂SO₄) is crucial in the manufacturing of batteries.
Memory Aids
Interactive tools to help you remember key concepts
Rhymes
In groups from 13 to 18, p-block elements included, diverse and keen!
Stories
Once upon a time in a chemistry lab, p-block elements formed a team; Nitrogen loved to preserve food, while Carbon dreamed of forming compounds. Oxygen came in to support combustion, and together they worked wonders in nature's rhythm.
Memory Tools
Remember 'C-N-O-S-Cl' to recall key p-block elements: Carbon, Nitrogen, Oxygen, Sulfur, and Chlorine.
Acronyms
P-BLOCK
'Prepare for chemistry - Be Logical
Observe
Create Knowledge!'
Flash Cards
Glossary
- pBlock Elements
Elements found in groups 13 to 18 of the periodic table characterized by having their outermost electrons in the p-orbital.
- Oxidation State
The degree of oxidation of an atom in a chemical compound, which can vary among p-block elements.
- Ammonia
A compound of nitrogen and hydrogen (NH₃) used in fertilizers and various chemical processes.
- Organic Chemistry
The branch of chemistry that deals with the structure, properties, and reactions of carbon-containing compounds.
- Allotropes
Different structural forms of the same element; e.g., diamond and graphite are allotropes of carbon.
- Ozone
A molecule composed of three oxygen atoms (O₃), known for its role in protecting Earth from UV radiation.
- Sulfuric Acid
A strong and widely used acid (H₂SO₄) in industrial processes and reactions.
- Halogens
Elements in group 17 of the periodic table, known for their high reactivity.
Reference links
Supplementary resources to enhance your learning experience.
