Formula for Sensible Heat
Enroll to start learning
You’ve not yet enrolled in this course. Please enroll for free to listen to audio lessons, classroom podcasts and take practice test.
Interactive Audio Lesson
Listen to a student-teacher conversation explaining the topic in a relatable way.
Understanding Sensible Heat
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Today, we're going to discuss sensible heat. Can anyone tell me what they understand by heat that causes a change in temperature?

I think it’s the heat that you feel, like when you touch something hot!

Exactly! Sensible heat is the heat that changes a substance's temperature, but it doesn’t change its state. Can someone else explain what that means?

So, it doesn't mean turning ice to water or water to steam?

Correct! That’s latent heat. Sensible heat influences how hot or cold something feels without changing its physical state. Remember the acronym **SHT**: Sensible Heat -> Temperature change.
Formula for Sensible Heat
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Now, let’s look at the formula used to calculate sensible heat. Can anyone recall the formula for specific heat?

Is it Q = mcΔT?

Great job! That’s the exact formula for sensible heat as well! Can anyone break it down for us?

Q is the heat energy, m is mass, c is specific heat capacity, and ΔT is the change in temperature.

Perfect! Remember this formula because it’s fundamental in thermodynamics. A mnemonic to help you could be **MCΔT** - like a cooking recipe: Mass, Capacity, and Temperature!
Applications of Sensible Heat
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Can anyone think of real-life situations where we encounter sensible heat?

Boiling water - it gets hotter without changing into steam immediately!

Exactly! Before any phase change occurs, the temperature of the water rises. Other examples include heating a pot of soup. Anyone else?

What about when baking? The oven heats the dough before it starts to rise.

Fantastic! Always think about sensible heat when dealing with temperature changes in cooking and heating.
Understanding Specific Heat Capacity's Role
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Let's explore specific heat capacity – why is this property important in our calculations?

Does it affect how much heat is needed to change the temperature?

Exactly right! Different substances require different amounts of heat to change temperature. How might that impact cooking times for different foods?

We'd need to cook some things longer than others, depending on their specific heats!

Yes! That’s a crucial insight. Always consider material differences in thermal applications - a good mnemonics tool is **HOTS**: How Often Things cook based on Specific heat.
Reviewing Sensible Heat Concepts
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Let’s review what we’ve learned about sensible heat. What key points come to mind?

Sensible heat changes temperature without changing phase!

And we calculate it using Q = mcΔT!

Correct on both counts! Always remember the relationships: heat, temperature change, and specific heat. It’s essential to grasp how substances behave thermally.
Introduction & Overview
Read summaries of the section's main ideas at different levels of detail.
Quick Overview
Standard
Sensible heat is explained in this section as the specific heat needed to change the temperature of a substance. The formula for calculating sensible heat is provided, emphasizing its dependence on mass, specific heat capacity, and temperature change.
Detailed
Formula for Sensible Heat
Sensible heat refers to the heat that results in a temperature change of a substance without altering its phase. It plays a crucial role in understanding heat transfer, as it describes how substances absorb and release heat when their temperatures change.
Key Points:
- Definition: Sensible heat is the amount of heat needed to raise or lower the temperature of a substance without a phase change.
- Formula: The formula for calculating sensible heat is the same as that for specific heat:
Q = mcΔT
Where:
- Q = Heat energy (in Joules)
- m = Mass of the substance (in kilograms)
- c = Specific heat capacity of the substance (in J/kg°C or J/kg·K)
- ΔT = Change in temperature (in °C or K)
Understanding sensible heat is essential for grasping how energy is transferred in thermal processes and the behavior of substances under thermal stress.
Youtube Videos


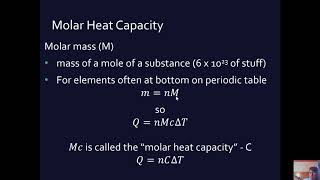





![What is Heat, Specific Heat & Heat Capacity in Physics? - [2-1-4]](https://img.youtube.com/vi/2tDKLkj9zfI/mqdefault.jpg)
Audio Book
Dive deep into the subject with an immersive audiobook experience.
Understanding Sensible Heat
Chapter 1 of 2
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Sensible heat is the heat that causes a change in temperature of a substance without a phase change. It is the heat required to raise or lower the temperature of a substance. Sensible heat depends on the mass, the specific heat capacity, and the change in temperature.
Detailed Explanation
Sensible heat refers to the heat energy that results in a temperature change of a substance. Unlike latent heat, which involves a change of state (like melting or boiling), sensible heat only changes the temperature. The amount of sensible heat depends on three main factors:
1. Mass of the substance - More mass means more heat is needed to change the temperature.
2. Specific heat capacity - This is a property of the material that indicates how much heat is required to raise its temperature.
3. Change in temperature (ΔT) - This is how much you want to change the temperature by.
The formula combines these factors to quantify the heat involved.
Examples & Analogies
Think of a cup of water. If you heat it on the stove, the temperature of the water increases. This increase in temperature is due to sensible heat. If you think of heating water on a stove: to get it from cold to hot signifies transferring sensible heat. However, if you leave the water on until it boils and turns into steam, that heat energy doesn’t change the temperature of water during that state change; it becomes latent heat.
Formula for Sensible Heat
Chapter 2 of 2
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
The formula for sensible heat is the same as the one for specific heat:
Q=mcΔT
Where:
○ QQ = Heat energy (in Joules)
○ mm = Mass of the substance (in kilograms)
○ cc = Specific heat capacity of the substance (in J/kg°C or J/kg·K)
○ ΔTΔT = Change in temperature (in °C or K)
Detailed Explanation
The formula for calculating sensible heat is: Q = mcΔT, where:
- Q represents the total heat energy transferred, measured in Joules.
- m is the mass of the substance, measured in kilograms.
- c is the specific heat capacity, which tells us how much heat energy is needed to raise the temperature of one kilogram of the substance by one degree Celsius (or Kelvin).
- ΔT is the change in temperature, calculated as the final temperature minus the initial temperature. This formula allows you to find the amount of heat required for a specific temperature change in a given mass of a substance.
Examples & Analogies
Imagine you are cooking pasta. You have 1 kg of water at room temperature (20°C), and you want to heat it to boiling (100°C). Using this formula, you can calculate how much heat is needed to raise the temperature of that water based on its mass and specific heat capacity. If water has a specific heat capacity of about 4.18 kJ/kg°C, you can plug in these values to find out how much energy you need to heat your pasta water.
Key Concepts
-
Sensible Heat: The heat that leads to a change in temperature without changing the phase of a substance.
-
Formula for Sensible Heat: Q = mcΔT, where Q represents heat, m is mass, c is specific heat capacity, and ΔT is the temperature change.
-
Specific Heat Capacity: The property that defines how much heat a substance can absorb or release to change its temperature.
Examples & Applications
Heating 2 kg of water from 20°C to 100°C requires calculating sensible heat using Q = mcΔT.
When heating a metal, the metal's temperature rises as heat is applied depending on its specific heat capacity.
Memory Aids
Interactive tools to help you remember key concepts
Rhymes
Sensible heat, oh so neat, changes temp, it’s no defeat!
Stories
Imagine turning ice into water—first, you warm it up. The heat makes it feel warm, but it doesn’t become water until enough heat is added.
Memory Tools
QMC ΔT: Quick Mass Change Temperature — remember to adjust mass, heat, and temperature!
Acronyms
Remember **SHC** for Sensible Heat Changes, where heat leads to temp shifts, not phases!
Flash Cards
Glossary
- Sensible Heat
The heat that causes a change in the temperature of a substance without a phase change.
- Specific Heat Capacity
The amount of heat required to raise the temperature of a unit mass of a substance by one degree Celsius.
- ΔT
Change in temperature, typically measured in degrees Celsius (°C) or Kelvin (K).
- Q
Heat energy measured in Joules (J).
- m
Mass of the substance, measured in kilograms (kg).
- c
Specific heat capacity of the substance, expressed in Joules per kilogram per degree Celsius (J/kg°C).
Reference links
Supplementary resources to enhance your learning experience.
