Introduction to Heat
Enroll to start learning
You’ve not yet enrolled in this course. Please enroll for free to listen to audio lessons, classroom podcasts and take practice test.
Interactive Audio Lesson
Listen to a student-teacher conversation explaining the topic in a relatable way.
Understanding Heat
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Today, we're going to discuss heat. Can anyone tell me what heat is?

I think it's the energy we feel when something's warm.

That's correct, but it's more specific! Heat is energy that flows from one body to another due to a temperature difference.

So, it's not a substance?

Exactly! Heat is the transfer of energy, not a thing we can hold. It is measured in Joules. Can anyone explain how heat might be transferred?

Through conduction, right? Like touching a hot stove!

Yes, that’s a great example of conduction! It’s the transfer of heat through direct contact.
Temperature vs Heat
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Let’s clarify the difference between temperature and heat. Can anyone tell me what temperature measures?

The average kinetic energy of particles?

Exactly! While temperature measures how fast particles are moving on average, heat measures the total energy transferred due to temperature differences. Does that make sense?

So, if I have a large pot of soup at a high temperature, it has a lot of heat?

Correct! Because it’s not just about how hot it is, but how much energy it contains altogether.
Methods of Heat Transfer
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Now let’s discuss the different methods of heat transfer. Can anyone name them?

I remember conduction, convection, and radiation!

Good job! Can you give an example of each?

Conduction is like when I touch something hot. Convection happens in boiling water, and radiation is the heat from the sun.

Perfect! Each method illustrates how heat interacts differently with various materials and environments. Very well done!
Heat Capacity and Specific Heat
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Finally, let’s touch on heat capacity and specific heat. Who can explain what specific heat is?

Isn’t it how much heat is needed to change the temperature of a substance?

Yes! It's the heat needed to raise the temperature of one kilogram of a substance by one degree Celsius. This varies among different materials. How do we express it?

In Joules per kilogram per degree Celsius, right?

Exactly! Specific heat capacity is crucial for understanding how different materials react to heat.
Introduction & Overview
Read summaries of the section's main ideas at different levels of detail.
Quick Overview
Standard
Heat is energy that flows from warmer to cooler bodies, resulting in temperature changes or phase transitions. It is measured in Joules, and is distinct from temperature, which quantifies the average kinetic energy of particles.
Detailed
Introduction to Heat
Heat, measured in Joules (J), is a form of energy that transfers from a higher temperature body to a lower temperature body. Unlike temperature, which reflects the average kinetic energy of particles within a substance, heat quantifies the total energy transferred due to temperature differences. The transfer can occur through three primary mechanisms: conduction, convection, and radiation, each important in various physical processes.
Key Points
- Heat Transfer: Always from hot to cold.
- Energy vs. Temperature: Heat is energy transfer; temperature indicates the energy per particle.
- Measurement: Heat is quantified in Joules (J).
- Methods of Transfer: Heat can be transferred in three ways: conduction, convection, and radiation.
Understanding heat is essential in fields ranging from physics to environmental science, impacting both theoretical frameworks and practical applications.
Youtube Videos


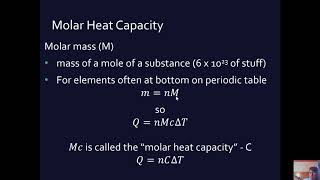





![What is Heat, Specific Heat & Heat Capacity in Physics? - [2-1-4]](https://img.youtube.com/vi/2tDKLkj9zfI/mqdefault.jpg)
Audio Book
Dive deep into the subject with an immersive audiobook experience.
What is Heat?
Chapter 1 of 2
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Heat is a form of energy that flows from a body at a higher temperature to a body at a lower temperature. It is transferred through conduction, convection, or radiation and results in a change in the temperature or state of the substance.
- Heat is measured in Joules (J) in the SI system.
- Heat is not a substance but a transfer of energy due to temperature differences.
Detailed Explanation
Heat is the energy that moves from warmer objects to cooler ones. This transfer happens in three ways: conduction (through direct contact), convection (through fluids like air or water), and radiation (through electromagnetic waves). It’s important to note that heat itself is not something you can hold; rather, it's a process of energy moving because of differences in temperature. It’s measured in Joules (J), a standard unit of energy in the International System of Units (SI).
Examples & Analogies
Think of heat like a crowd in a concert venue. People on the dance floor (higher temperature) might move towards the cooler areas (like the back of the venue) when they start to feel too hot. The movement of people represents the flow of energy, similar to how heat moves from hot to cold areas. Just as the number of people can change the dynamics of the venue, temperature differences lead to the transfer of heat energy.
Heat and Temperature
Chapter 2 of 2
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
While temperature measures the average kinetic energy of particles, heat measures the total energy transferred due to temperature differences. The amount of heat transferred depends on the mass, the specific heat capacity of the substance, and the temperature change.
Detailed Explanation
Temperature is a measure of how fast the particles in a substance are moving on average. In contrast, heat quantifies the total energy being transferred from one place to another because of temperature differences. The total amount of heat that flows depends on several factors: the mass of the substance involved, the specific heat capacity (how much heat is needed to raise the temperature of a specific mass of the substance by 1 degree), and how much the temperature changes.
Examples & Analogies
Imagine boiling water for tea. The temperature of the water starts at room temperature and gradually increases as you heat it. The heat from the stove warms the water, and the warmer water can eventually boil. Just as it takes more time to warm a large pot of water compared to a small cup, the mass and specific heat capacity of the water will affect how much heat energy is required to change its temperature.
Key Concepts
-
Heat: Energy transferred due to temperature difference.
-
Temperature: Average kinetic energy of particles in a substance.
-
Conduction: Heat transfer through contact.
-
Convection: Heat transfer through fluid movement.
-
Radiation: Heat transfer through electromagnetic waves.
-
Specific Heat Capacity: Heat needed to change the temperature of one kilogram of substance by one degree Celsius.
Examples & Applications
Heating soup on a stove involves conduction as the pot contacts the heat source.
Boiling water illustrates convection, as warm water rises and cooler water sinks.
The sun warming the Earth is an example of heat transfer by radiation.
Memory Aids
Interactive tools to help you remember key concepts
Rhymes
Heat flows from hot to cold, that's the rule that must be told.
Stories
Imagine a sunny day where the sun’s rays travel through space, warming everything in their path—this illustrates how radiation works.
Memory Tools
Conduction, convection, and radiation—remember 'C-C-R' to recall the three methods of heat transfer.
Acronyms
H.E.A.T. – Heat Energy Always Travels (from hot to cold).
Flash Cards
Glossary
- Heat
Energy that flows from a higher temperature body to a lower temperature body.
- Temperature
The measure of the average kinetic energy of particles in a substance.
- Conduction
The transfer of heat through direct contact between materials.
- Convection
The transfer of heat in a fluid (liquid or gas) through the movement of the fluid.
- Radiation
The transfer of heat through electromagnetic waves without requiring a medium.
- Specific Heat Capacity
The amount of heat required to raise the temperature of one kilogram of a substance by one degree Celsius.
- Joules
The SI unit of measurement for energy, including heat.
Reference links
Supplementary resources to enhance your learning experience.
