Cyclic process
Enroll to start learning
You’ve not yet enrolled in this course. Please enroll for free to listen to audio lessons, classroom podcasts and take practice test.
Interactive Audio Lesson
Listen to a student-teacher conversation explaining the topic in a relatable way.
Introduction to Cyclic Processes
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Today, we're going to learn about cyclic processes in thermodynamics. Can anyone tell me what happens to a thermodynamic system in a cyclic process?

I think it returns to its original state.

Correct! When a system undergoes a cyclic process, it indeed returns to its original state. This means that there is no overall change in the internal energy of the system. Can anyone explain why?

Because internal energy is a state function, and it is defined only by the initial and final states.

Exactly! Since internal energy depends only on the state, ΔU equals zero at the end of the cycle. Remember this: 'State Functions Never Change in a Cycle' — it's a handy mnemonic!

What about the heat and work done during this process?

Great question! Because of the First Law of Thermodynamics, we know that ΔQ, which is the heat absorbed, equals the work done by the system, ΔW. Let's summarize: in a cyclic process, ΔU = 0, which means ΔQ = ΔW.
Implications of Cyclic Processes
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Now that we have an understanding of cyclic processes, can someone share where we might encounter these cycles in real life?

Maybe in engines, like car engines or steam engines?

Absolutely! Car engines use cyclic processes to convert heat into work. The heat from fuel combustion causes the gas to expand, pushing the piston and doing work. What about refrigerators? How do they relate?

Refrigerators also use cyclic processes to remove heat from the inside and keep it cold.

Yes! They absorb heat from the food compartment and release it outside the refrigerator. In this process too, the system goes through various changes but returns to its original state. Always remember, whether in engines or refrigerators, they rely on cyclic processes to function efficiently.

So the work done is equal to the heat replaced after a full cycle?

Precisely! ΔQ = ΔW indicates the energy balance in cyclic processes, which is crucial for understanding efficiency.
Mathematical Representation
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Let's delve into the mathematical side of cyclic processes. Can someone summarize how we express the energy relationship behind cyclic processes?

ΔU = 0 means ΔQ = ΔW at the end of the cycle.

Correct! And we can express it mathematically. When dealing with the cycle in engines, we calculate heat absorbed and work done across various stages. Can anyone think of an equation we could write down?

We could use the First Law of Thermodynamics: ΔQ = ΔU + ΔW.

Exactly! When we combine this with the fact that ΔU = 0 in a cyclic process, it confirms that ΔQ = ΔW during the cycle. This helps us analyze engine efficiency or refrigeration effectiveness. Keep practicing these equations, as they'll come in handy for solving problems!

Can we apply these concepts to analyze the efficiency of engines?

Very insightful! Understanding ΔQ = ΔW is foundational for calculating engine efficiency and their operational mechanisms.
Introduction & Overview
Read summaries of the section's main ideas at different levels of detail.
Quick Overview
Standard
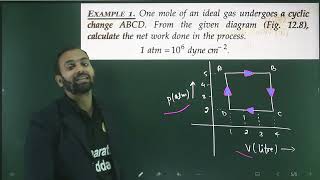
The cyclic process is a vital concept in thermodynamics, where the system undergoes various transformations and eventually returns to its original state. Since internal energy is a state function, it remains constant throughout the cycle, and the total heat added to the system equals the work done by the system.
Detailed
Cyclic Process in Thermodynamics
In thermodynamics, a cyclic process is defined as a sequence of transformations that a system undergoes before returning to its initial state. At the end of these transformations, all the state variables of the system — including internal energy, temperature, pressure, and volume — return to their original values. This implies that the change in internal energy, ΔU, over one complete cycle is equal to zero:
ΔU = 0.
From the First Law of Thermodynamics, we can express this as:
ΔQ = ΔU + ΔW, where ΔQ is the total heat added, and ΔW is the work done by the system during the cycle. Given that ΔU = 0, it follows that the total heat absorbed by the system must equal the work done by the system:
ΔQ = ΔW.
This relationship signifies a balance between energy input and output in cyclic processes, such as those found in engines or refrigerators, where energy circulates but ultimately returns to the original state during a full cycle. Understanding this concept is crucial for analyzing the efficiency and performance of thermodynamic systems.
Youtube Videos


Audio Book
Dive deep into the subject with an immersive audiobook experience.
Definition of a Cyclic Process
Chapter 1 of 2
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
In a cyclic process, the system returns to its initial state. Since internal energy is a state variable, ∆U = 0 for a cyclic process.
Detailed Explanation
A cyclic process refers to a situation in thermodynamics where a system goes through a series of processes and eventually returns to its original state, meaning all macroscopic properties of the system are restored to their initial values. In this context, the internal energy (∆U), which is a state variable, does not change over one complete cycle, thus ∆U = 0. The internal energy being zero indicates that any energy added to the system is fully converted into work or released as heat.
Examples & Analogies
Think of a car driving in a circular track. No matter how far the car travels around the track, when it returns to the starting point, it has not changed its position in space. Similarly, in a cyclic thermodynamic process, although the system undergoes various transformations, it finishes at the same energy state it began with.
Total Heat and Work in a Cyclic Process
Chapter 2 of 2
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
From Eq. (11.1), the total heat absorbed equals the work done by the system.
Detailed Explanation
In a cyclic process, the First Law of Thermodynamics implies that the total heat (Q) absorbed during the cycle is equal to the total work (W) done by the system. This relationship is derived from the equation ∆Q = ∆U + ∆W. Since ∆U is zero for a full cycle (as discussed earlier), it simplifies to Q = W, meaning that everything that is added to the system as heat is used to do work. Thus, the net change in energy across the entire cycle equals zero, signifying that energy is conserved.
Examples & Analogies
Consider a wind-up toy. When you twist the key to wind it up, you're adding energy (heat) to the toy. As you release it, that energy converts into motion (work). Once it stops, all energy added is used up in movement, and when the toy is reset (wound up again), it returns to its original 'state,' similar to returning to its starting point in a cyclic process.
Key Concepts
-
Cyclic Process: A system returns to its initial state, making internal energy change zero.
-
Internal Energy: This state function represents the total energy within a system, defined by its state variables.
-
First Law of Thermodynamics: Energy conservation—it equates the heat added to the work done in closed systems.
Examples & Applications
In a steam engine, water is heated to produce steam, which expands, driving a piston. After the work is done, it cools back down to return to the water state, completing a cycle.
In refrigerators, the refrigerant absorbs heat from the internal compartment and releases it to the external environment, going through cycles that maintain desired temperatures.
Memory Aids
Interactive tools to help you remember key concepts
Rhymes
In cycles we go, back to the start, Internal energy's balance, is a key part.
Stories
Imagine a bicycle journey through a park. You start at one point, ride around a loop, and come back to the same spot, similar to how a cyclic process works in thermodynamics.
Memory Tools
CYCLE: Constant Yield, Constant Laws of Energy.
Acronyms
C = Cyclic, Y = Yield, L = Laws, E = Energy.
Flash Cards
Glossary
- Cyclic Process
A thermodynamic process in which a system returns to its original state after a sequence of transformations.
- Internal Energy (U)
The total energy contained within a system, determined by the state variables such as temperature, volume, and pressure.
- First Law of Thermodynamics
A principle stating that energy cannot be created or destroyed, only transformed from one form to another.
- ΔQ
The heat added to or removed from a system.
- ΔW
The work done by or on the system during a thermodynamic process.
Reference links
Supplementary resources to enhance your learning experience.
