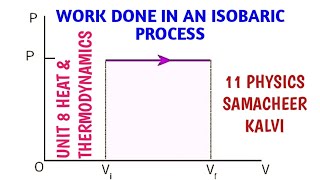
Isobaric process
Enroll to start learning
You’ve not yet enrolled in this course. Please enroll for free to listen to audio lessons, classroom podcasts and take practice test.
Interactive Audio Lesson
Listen to a student-teacher conversation explaining the topic in a relatable way.
Introduction to Isobaric Process
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Today we are going to discuss the isobaric process. Can anyone tell me what this process means?

Is it when the pressure of a system is constant?

Exactly! In an isobaric process, the pressure remains constant while the volume and temperature can change. That means when the gas expands, it does work on the surroundings.

How do we calculate the work done in an isobaric process?

Good question! The work done is calculated as W = P*(V2 - V1). Remember, since pressure is constant, it simplifies our calculations.

So if the volume increases, the gas does work, right?

That’s correct! And this can also be expressed in terms of temperature changes using the ideal gas law.

To summarize, in an isobaric process, pressure is constant, work is directly related to volume change, and heat transfer is involved!
Heat Transfer and Heat Capacity
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Now, let’s focus on heat transfer during isobaric processes. Can anyone remind me what heat capacity means?

Heat capacity is how much heat is needed to change the temperature of a substance.

Right! In an isobaric process, the heat added contributes to raising the temperature and doing work. This is described with the specific heat capacity at constant pressure.

What happens if more heat is added?

If you add more heat, both the internal energy and the work done will increase, which can lead to larger changes in volume or temperature.

So in practical applications, how do engineers use this?

Great question! Engineers apply this principle in various thermal systems such as heat engines where maintaining constant pressure while varying volume is crucial.

In summary, heat transfer is significant in isobaric processes, and understanding heat capacity at constant pressure is essential for practical applications.
Applications of Isobaric Processes
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Can anyone give examples of where we see isobaric processes in real life?

How about in car engines?

Exactly! Car engines often involve isobaric processes when the pressure remains constant during the combustion of fuel.

What about refrigerants?

Yes, in refrigeration cycles, isobaric processes occur when heat is absorbed or released at constant pressure, which helps in cooling.

So how do we measure the efficiency of these processes?

We can analyze work done and heat transfer to calculate efficiency. Efficiency is crucial in assessing the performance of engines or refrigerators.

To summarize, isobaric processes are vital in many applications, especially in engines and HVAC systems, as they involve constant pressure scenarios where heat and work interplay.
Introduction & Overview
Read summaries of the section's main ideas at different levels of detail.
Quick Overview
Standard
In an isobaric process, the pressure of a system does not change, which allows for the gas to either absorb heat or do work while experiencing changes in volume and temperature. This process is significant in applications such as heating and cooling systems.
Detailed
Isobaric Process
An isobaric process is a type of thermodynamic process in which pressure remains constant (denoted as P = constant) throughout the entire process. This is significant because it allows one to directly relate heat fluctuation to changes in volume and temperature. In an isobaric process, the initial and final states of a gas are characterized by constant pressure.
Key Points:
-
Work Done by the Gas: The work done during an isobaric process can be calculated using the formula:
W = P(V2 − V1) = µR(T2 − T1),
where W is the work performed, P is pressure, V2 and V1 are the final and initial volumes respectively, and T2 and T1 are the final and initial temperatures. - Heat Transfer and Internal Energy: In an isobaric process, the heat absorbed by the system contributes to both the increase in internal energy and the work done by the system. Hence, the change in temperature for a specific amount of heat is determined by the specific heat capacity at constant pressure.
The context of the isobaric process is commonly related to practical systems such as heating and cooling processes in engines and refrigeration, where the volume of gas changes while pressure remains unchanged. Thus, understanding isobaric processes provides insight into thermal dynamics used in everyday mechanical systems.
Youtube Videos






Audio Book
Dive deep into the subject with an immersive audiobook experience.
Definition of Isobaric Process
Chapter 1 of 2
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
In an isobaric process, P is fixed. W ork done by the gas is
W = P (V2 – V1) = µ R (T2 – T1) (11 .17)
Detailed Explanation
An isobaric process is one in which the pressure of the gas remains constant while its volume and temperature may change. The work done by the gas can be calculated using the formula W = P (V2 - V1), where P is the constant pressure, and V1 and V2 are the initial and final volumes of the gas, respectively. Since temperature changes throughout the process, it affects the internal energy of the gas, making this process essential in understanding how gases behave under various conditions.
Examples & Analogies
Imagine a balloon being slowly inflated to exactly match the weight of air outside it. As it expands, the pressure inside the balloon remains constant (assuming it's done carefully). You can think of this as the balloon 'pushing against' the atmosphere. The work done to inflate the balloon (expanding it) is equivalent to the pressure multiplied by the change in volume of the balloon.
Implications of Temperature Change
Chapter 2 of 2
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Since temperature changes, so does internal energy. The heat absorbed goes partly to increase internal energy and partly to do work. The change in temperature for a given amount of heat is determined by the specific heat of the gas at constant pressure.
Detailed Explanation
When the temperature of the gas increases during an isobaric process, the internal energy of the gas must also increase. This is because the heat added to the gas serves two purposes: it increases the internal energy and performs work by causing the gas to expand against a constant external pressure. To quantify how much the temperature increases per unit heat absorbed, we use the specific heat of the gas at constant pressure (Cp). This value tells us how much heat is needed to raise the temperature of a given amount of gas.
Examples & Analogies
Consider warming water in a pot on the stove. The water expands slightly as it heats up (increasing its volume) and turns into steam. In doing so, it needs energy (heat from the stove) to increase its temperature. Some of this heat energy contributes to increasing the internal energy of the water as well as performing work as it pushes against the atmosphere to create steam.
Key Concepts
-
Constant Pressure: In an isobaric process, the pressure of the gas does not change.
-
Work Done Calculation: Work done during an isobaric process is W = P(V2 - V1).
-
Heat Transfer: In this process, heat added raises both internal energy and does work.
Examples & Applications
Example of an isobaric process includes the heating of a gas at constant pressure in a piston-cylinder arrangement where the gas expands.
In a refrigerator, the refrigerant undergoes isobaric processes to absorb and reject heat.
Memory Aids
Interactive tools to help you remember key concepts
Rhymes
Isobaric, pressure's the key, work and heat, flow free.
Stories
Imagine a balloon at a party. As it's heated, it expands but doesn’t pop—this is an isobaric process where pressure remains constant.
Memory Tools
Pursue Constant Pressure: 'Isobaric' means pressure doesn’t budge!
Acronyms
ISOBAR
'I Stay On Bar Pressure' - remember isobaric keeps pressure constant.
Flash Cards
Glossary
- Isobaric Process
A thermodynamic process where the pressure remains constant while the volume and temperature of the system may change.
- Work Done (W)
The energy transferred when a force is applied, calculated in an isobaric process as W = P(V2 - V1).
- Specific Heat Capacity at Constant Pressure (Cp)
The amount of heat required to raise the temperature of a unit mass of a substance at constant pressure.
Reference links
Supplementary resources to enhance your learning experience.
