THERMODYNAMIC PROCESSES
Enroll to start learning
You’ve not yet enrolled in this course. Please enroll for free to listen to audio lessons, classroom podcasts and take practice test.
Interactive Audio Lesson
Listen to a student-teacher conversation explaining the topic in a relatable way.
Quasi-static Processes
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Today, we are going to explore quasi-static processes. These idealized processes occur so slowly that the system remains in equilibrium with its surroundings. Can anyone think of a real-world example of a quasi-static process?

Maybe the slow inflation of a balloon?

Good example! The slower you inflate it, the more likely the gas inside remains at a uniform pressure and temperature. How is this beneficial?

It helps ensure there are no sudden changes that could burst it!

Exactly! Memory aid: Think 'Slow & Steady (S&S) keeps it steady' for quasi-static processes. They are crucial for understanding thermodynamics.

So, are all processes quasi-static?

No, not all! But they provide a useful model. Let's recap: Quasi-static processes keep the system at equilibrium so that analysis aligns with ideal thermodynamic laws.
Isothermal Processes
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Let's move to isothermal processes. What does it imply when we say a process is isothermal?

It means the temperature doesn't change during the process, right?

Correct! And thus, if a gas is expanding isothermally, how are pressure and volume related?

According to Boyle’s Law, pressure decreases as volume increases!

Exactly! A mnemonic to remember this: 'Pressure and Volume are Friends (PV = constant)' in isothermal processes. Why is this significance?

It helps us design engines and other equipment that maximize efficiency!

Well said! Reviewing key points: An isothermal process keeps temperature constant and relates pressure and volume inversely.
Adiabatic Processes
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Now, we'll tackle adiabatic processes. What characterizes them?

No heat is exchanged with the surroundings!

Exactly! That leads to changes in internal energy due to work done. Can someone explain how this affects temperature?

When the gas expands, it does work which leads to a drop in temperature!

Fantastic! Think of a memory aid: 'Adiabatic = No heat, Adventure in Energy' to highlight the work involved changing the internal state. What about pressure?

Adiabatic processes follow PV^γ = constant! That shows how pressure and volume relate.

Spot on! Let's recap: In an adiabatic process, temperature changes as heat isn't exchanged, forming essential thermodynamic relationships.
Isochoric and Isobaric Processes
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Moving on to isochoric and isobaric processes! What is unique about each?

An isochoric process has constant volume, so no work is done!

Meanwhile, an isobaric process maintains constant pressure, allowing for work done as volume changes!

Precisely! For isochoric, heat adds to internal energy directly. Remember: 'Isochoric = No Work, Heat = Internal Energy'. What that's mean mathematically?

That means all the heat added goes straight into raising temperature!

Correct! While for isobaric, heat absorbed splits between work done and internal energy change. Catchphrase: 'Isobaric = Pressure on the Rise'—conveys key ideas!

Perfect, so many processes connect back to First Law principles!

Exactly! Each process defines energy movement and state shifts we apply in everyday thermodynamic applications.
Cyclic Processes
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Now, let’s talk about cyclic processes. What does it mean for a system to be in a cyclic process?

It means the system returns to its initial state after completing a series of processes!

Right! And since the internal energy is a state variable, what does that say about the internal energy change in a cyclic process?

The change is zero; all energy is converted to work or heat through the cycle!

Exactly! A memory aid for cyclic processes could be: 'Cycle of Energy, Loop of Work'. So how do we relate these ideas to engines?

Cyclic processes are fundamental to engine functions since they allow energy transfer in a controlled manner.

Exactly! To summarize: In a cyclic process, the system’s internal energy returns to its initial state, emphasizing energy conservation.
Introduction & Overview
Read summaries of the section's main ideas at different levels of detail.
Quick Overview
Standard
Thermodynamic processes illustrate how systems change states under different constraints, focusing on isothermal, adiabatic, isochoric, and isobaric processes. Each process has unique characteristics that determine how energy is transferred, work is done, and temperatures change, which helps in understanding the behavior of thermodynamic systems.
Detailed
Thermodynamic Processes
Thermodynamic processes refer to the changes in a system's internal state, characterized by heat and work interactions under different constraints. Each process dictates how pressure, volume, and temperature interact over time.
1. Definitions
- Quasi-static process: An ideally slow process through which a system remains in consistent thermal and mechanical equilibrium. Changes occur so gradually that the system is virtually at equilibrium at all times.
- Isothermal process: The system’s temperature remains constant as the gas expands or contracts. According to Boyle's Law, the pressure and volume of an ideal gas are inversely related: PV = constant.
- Adiabatic process: No heat is exchanged with the surroundings. The internal energy of the system changes solely due to work done; thus, the temperature and pressure can change in a non-linear fashion.
- Isochoric process: Volume remains constant, meaning that no work is done; all the heat transferred contributes to the change in internal energy.
- Isobaric process: The pressure remains constant. Work is done by the system or on the system, leading to a change in volume and temperature related to the heat transferred.
- Cyclic process: The system returns to its initial state, resulting in no change in internal energy. The total heat absorbed during the cycle equals the total work done.
2. Significance
Understanding these processes allows for the application of the First Law of Thermodynamics, where energy conservation principles define the behavior of thermodynamic systems, making it vital for engines, refrigerators, and other real-world applications.
Youtube Videos





Audio Book
Dive deep into the subject with an immersive audiobook experience.
Quasi-static Process
Chapter 1 of 5
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Consider a gas in thermal and mechanical equilibrium with its surroundings. The pressure of the gas in that case equals the external pressure and its temperature is the same as that of its surroundings. Suppose that the external pressure is suddenly reduced (say by lifting the weight on the movable piston in the container). The piston will accelerate outward. During the process, the gas passes through states that are not equilibrium states. The non-equilibrium states do not have well-defined pressure and temperature. In the same way, if a finite temperature difference exists between the gas and its surroundings, there will be a rapid exchange of heat during which the gas will pass through non-equilibrium states.
Detailed Explanation
A quasi-static process occurs when the system changes so slowly that it remains in thermal and mechanical equilibrium with its surroundings at each step. This means that the pressure and temperature of the gas remain infinitesimally different from that of the environment as changes are made. If the pressure is suddenly reduced, the piston moves rapidly, and the gas may not equilibrate, leading to undefined values of pressure and temperature during this rapid transition. This is unlike a quasi-static process where every infinitesimal change keeps the gas in equilibrium, allowing for well-defined pressures and temperatures throughout the process.
Examples & Analogies
Imagine filling a balloon with air. If you blow air into the balloon steadily and slowly, the balloon expands uniformly, representing a quasi-static process where the pressure inside the balloon matches the external pressure. Conversely, if you were to quickly exhale hard into the balloon, it would expand suddenly and not uniformly throughout its length, creating regions of varying pressures and temperatures rather than an even distribution.
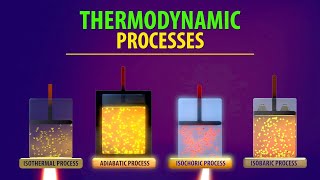
Types of Thermodynamic Processes
Chapter 2 of 5
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
A process in which the temperature of the system is kept fixed throughout is called an isothermal process. The expansion of a gas in a metallic cylinder placed in a large reservoir of fixed temperature is an example of an isothermal process. In isobaric processes the pressure is constant while in isochoric processes the volume is constant. Finally, if the system is insulated from the surroundings and no heat flows between the system and the surroundings, the process is adiabatic.
Detailed Explanation
Thermodynamic processes can be classified into several types based on their constraints:
1. Isothermal Process: Here, the temperature remains constant. The gas absorbs or releases heat while doing work, maintaining thermal equilibrium with a heat reservoir.
2. Isobaric Process: This process occurs at a constant pressure. When a gas expands or contracts under a constant external pressure, its volume changes while its pressure stays the same.
3. Isochoric Process: Volume remains constant during this type of process, which means no work is done by or on the system. All the supplied heat energy goes into changing the internal energy, thereby increasing the temperature.
4. Adiabatic Process: No heat is exchanged with the surroundings, meaning all energy transfers result from work done on or by the gas, leading to temperature changes without heat exchange.
Examples & Analogies
Think of a pressure cooker. It maintains a constant pressure (isobaric) while cooking food. If you say a pot of water is kept on a stove at a constant temperature (isothermal) while boiling, you can also consider it an isochoric process if the lid is tightly closed, preventing any steam from escaping. In contrast, during its operation, if you rapidly stir the water in a sealed container (adiabatic), it heats up due to the work done on it without heat exchange with the environment.
Isothermal Process
Chapter 3 of 5
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
For an isothermal process (T fixed), the ideal gas equation gives PV = constant, i.e., pressure of a given mass of gas varies inversely as its volume. Suppose an ideal gas goes isothermally (at temperature T) from its initial state (P1, V1) to the final state (P2, V2).
Detailed Explanation
In an isothermal process, the product of pressure and volume for a gas remains constant because the temperature does not change. According to Boyle's Law, if the volume increases, the pressure decreases and vice-versa, as long as the temperature is held constant. So for a gas going from an initial condition of (P1, V1) to a final condition of (P2, V2), the relationship can be expressed by the equation PV = constant. Mathematically, as the gas expands or compresses while keeping temperature constant, its state can be described by this inverse relationship.
Examples & Analogies
A practical analogy would be using a syringe. If you pull back on the plunger (increasing the volume), the pressure inside the syringe decreases. If you push in the plunger without causing the liquid to heat up (isothermal condition), the pressure increases as the volume decreases. No matter how much you change the volume, as long as the liquid remains at a constant temperature, the product of pressure and volume will always equal a constant.
Adiabatic Process
Chapter 4 of 5
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
In an adiabatic process, the system is insulated from the surroundings and heat absorbed or released is zero. From Eq. (11.1), we see that work done by the gas results in decrease in its internal energy (and hence its temperature for an ideal gas).
Detailed Explanation
During an adiabatic process, since the system is perfectly insulated, no heat is exchanged with the surroundings. This means any work done by the system (like expanding gases doing work on pistons) reduces its internal energy and results in a decrease in temperature. Conversely, if work is done on the gas (compressing it), its internal energy and therefore temperature increases. This is distinctly different from processes where heat can enter or exit the system.
Examples & Analogies
Think of a bicycle pump. When you compress the air inside the pump, you feel the pump heating up (the work done on the gas increases its temperature)—that's the adiabatic process at work. If the pump were insulated, not only would the air within get hot as you compress it, but if you were to let the gas expand without allowing heat to escape, it would cool down as it expands, demonstrating that work translates directly into internal energy changes.
Cyclic Process
Chapter 5 of 5
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
In a cyclic process, the system returns to its initial state. Since internal energy is a state variable, ∆U = 0 for a cyclic process. From Eq. (11.1), the total heat absorbed equals the work done by the system.
Detailed Explanation
In a cyclic process, the system experiences various transitions but ultimately ends up in the exact same state it began with. Because internal energy is a state variable, the change in internal energy (∆U) during a complete cycle is zero, meaning any energy put in as heat must be transformed into work done by the system. The First Law of Thermodynamics (∆Q = ∆U + ∆W) simplifies here since ∆U is zero, leading to ∆Q = ∆W, indicating all heat added goes into doing work.
Examples & Analogies
Consider a car engine operating on a four-stroke cycle. The engine takes in fuel and mixes it with air (intake), compresses the mixture (compression), ignites it to perform work (power), and expels exhaust gases (exhaust). After completing these four strokes, the process repeats. The engine returns to its starting point at the end of each cycle, meaning the energy transformations in ∆U are reset, while the total energy used remains accountable for work done in that cycle.
Key Concepts
-
Quasi-static Process: Maintains equilibrium throughout and helps understand thermodynamic laws.
-
Isothermal Process: Temperature remains constant, relates pressure and volume inversely.
-
Adiabatic Process: No heat exchange; temperature changes due to work done.
-
Isochoric Process: Volume constant; internal energy increases with heat added.
-
Isobaric Process: Pressure constant; work is done as volume changes.
-
Cyclic Process: System returns to initial state, emphasizing energy conservation.
Examples & Applications
A gas expanding in a slow, controlled manner in a piston-cylinder setup represents a quasi-static process.
Heating water at a constant temperature while it evaporates is an example of an isothermal process.
An insulated gas expanding in a vacuum demonstrates an adiabatic process.
A sealed container of gas whose volume cannot change exemplifies an isochoric process.
A gas in a constant-pressure cooker while boiling water showcases an isobaric process.
A car engine completing its operating cycle is an example of a cyclic process.
Memory Aids
Interactive tools to help you remember key concepts
Rhymes
In a quasi-static flow, equilibrium shall grow.
Stories
Imagine a gas bath where the temperature's just right, it expands and relaxes all day and night—that's isothermal bliss!
Memory Tools
Adiabatic = Absence of heat; remember, work is my only treat.
Acronyms
C.I.S.A.
for Cyclic
for Isochoric
for Isothermal
for Adiabatic.
Flash Cards
Glossary
- QuasiStatic Process
A process that occurs slowly enough to maintain thermal and mechanical equilibrium.
- Isothermal Process
A thermodynamic process in which the temperature remains constant.
- Adiabatic Process
A thermodynamic process with no heat exchange with the surroundings.
- Isochoric Process
A process where the volume of the system remains constant, implying no work is done.
- Isobaric Process
A process in which the pressure is held constant while volume and temperature may change.
- Cyclic Process
A thermodynamic process that returns to its original state, resulting in no change in internal energy.
Reference links
Supplementary resources to enhance your learning experience.
