Quasi-static process
Enroll to start learning
You’ve not yet enrolled in this course. Please enroll for free to listen to audio lessons, classroom podcasts and take practice test.
Interactive Audio Lesson
Listen to a student-teacher conversation explaining the topic in a relatable way.
Introduction to Quasi-static Processes
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Today, we will cover quasi-static processes. Can anyone tell me what they think a quasi-static process is?

Isn’t it a process that happens very slowly, so that the system stays in equilibrium?

Exactly! A quasi-static process occurs so slowly that the system remains in thermal and mechanical equilibrium with its surroundings. This is important for applying thermodynamic laws. Can you think of an example?

Maybe when a gas expands very slowly in a piston?

Correct! When a gas expands slowly in a piston, it maintains equilibrium, allowing us to apply equations more accurately.

How does it differ from a regular expansion?

Great question! In a regular expansion, the system may pass through non-equilibrium states, which complicates the analysis. Remember, in quasi-static processes, the changes are infinitesimal, ensuring equilibrium at every moment. Let’s summarize: Quasi-static processes are idealized, infinitely slow changes maintaining equilibrium.
Characteristics of Quasi-static Processes
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Now, let’s delve into some characteristics of quasi-static processes. What do you think happens to pressure during these processes?

I think the pressure difference between the system and surroundings is very small.

Absolutely right! In a quasi-static process, the pressure difference is infinitesimally small. What about temperature?

Is it the same? The temperature difference is also very small?

Yes! The temperature of the system and surroundings must also differ only infinitesimally. This allows us to treat the system and surroundings as if they were always at equilibrium.

Are there any other types of processes related to this idea?

Great point! There are several types, like isothermal, adiabatic, isochoric, and isobaric processes, all of which can be seen as quasi-static if the changes are sufficiently slow. For example, an isothermal process means that temperature remains constant while the process is quasi-static.

So, these processes are foundational for understanding thermodynamics?

Exactly! Quasi-static processes play a crucial role in thermodynamic discussions, making them essential for grasping more complex concepts. Let's summarize: Key features of quasi-static processes include infinitesimal differences in pressure and temperature, maintaining equilibrium.
Introduction & Overview
Read summaries of the section's main ideas at different levels of detail.
Quick Overview
Standard
In a quasi-static process, a thermodynamic system undergoes changes infinitely slowly, allowing it to maintain thermal and mechanical equilibrium with its surroundings. This concept is crucial for understanding various thermodynamic processes such as isothermal, adiabatic, isochoric, and isobaric processes.
Detailed
Quasi-static Process
A quasi-static process is a theoretical construct in thermodynamics where the system changes its state at an infinitely slow pace. This allows the system to remain in thermal and mechanical equilibrium with its surroundings at all times. In contrast, non-quasi-static processes involve rapid changes that result in the system passing through a series of non-equilibrium states, which do not possess well-defined pressure and temperature.
Key Characteristics
- During a quasi-static process, the pressure difference between the system and the external environment is infinitesimally small.
- Similarly, the temperature difference between the system and its surroundings is also infinitesimal.
- Such processes are hypothetical but serve as an excellent approximation for many real-world situations when changes occur slowly.
- Examples include processes like isothermal expansion, adiabatic compression, isochoric processes, and isobaric processes.
In practical applications, processes that can be approximated as quasi-static are helpful in accurately applying concepts of thermodynamics, where the system's variables remain well-defined throughout the changes.
Youtube Videos



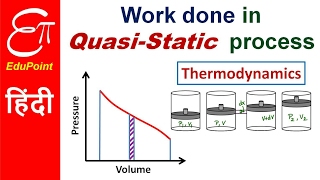


Audio Book
Dive deep into the subject with an immersive audiobook experience.
Definition of Quasi-static Process
Chapter 1 of 4
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Consider a gas in thermal and mechanical equilibrium with its surroundings. The pressure of the gas in that case equals the external pressure and its temperature is the same as that of its surroundings.
Detailed Explanation
A quasi-static process refers to a thermodynamic process that occurs so slowly that the system remains in equilibrium with its surroundings throughout the entire process. This means that at each moment, the pressure and temperature of the system are equal to those of the surrounding environment. If you imagine a gas in a piston, for instance, the pressure of the gas adjusts gradually as the piston moves very slowly, allowing the system to adapt and remain in equilibrium.
Examples & Analogies
Think about slowly squeezing a sponge filled with water. If you press it gently, the water inside shifts gradually and uniformly, maintaining an equilibrium throughout. But if you squeeze it suddenly and forcefully, water might burst out in an uncontrolled manner, akin to a non-quasi-static process.
Consequences of Non-equilibrium States
Chapter 2 of 4
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Suppose that the external pressure is suddenly reduced (say by lifting the weight on the movable piston in the container). The piston will accelerate outward. During the process, the gas passes through states that are not equilibrium states. The non-equilibrium states do not have well-defined pressure and temperature.
Detailed Explanation
If the external pressure acting on the gas in the piston is suddenly decreased, the piston will move quickly outward due to the increased pressure difference. During this rapid expansion, the gas does not have time to adjust and reach an equilibrium state. It will pass through a series of non-equilibrium states where pressure and temperature are not well defined. This means that measurements of pressure and temperature could fluctuate wildly during this process.
Examples & Analogies
Imagine a balloon being suddenly released from a person’s grip. The air inside the balloon rushes out quickly. The changes in pressure and temperature inside the balloon aren’t uniform, and one could say that the balloon is going through various non-equilibrium states until the air is completely gone.
Reaching Equilibrium
Chapter 3 of 4
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
In due course, the gas will settle to an equilibrium state with well-defined temperature and pressure equal to those of the surroundings.
Detailed Explanation
After undergoing the rapid changes and passing through non-equilibrium states, the gas will eventually return to a state of equilibrium. This means that it will stabilize at a pressure and temperature that match those of its surroundings. At this point, all measurements of its variables will become predictable and consistent.
Examples & Analogies
Think of a person jumping into a swimming pool. Initially, they create chaos in the water, splashing around (non-equilibrium state). However, after a while, the water settles down, the surface becomes calm again, and the temperature of the water will equalize throughout – this reflects reaching an equilibrium state.
The Importance of Quasi-static Processes
Chapter 4 of 4
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
It is, therefore, convenient to imagine an idealized process in which at every stage the system is an equilibrium state. Such a process is, in principle, infinitely slow, hence the name quasi-static (meaning nearly static).
Detailed Explanation
Quasi-static processes are crucial in thermodynamics as they represent the ideal conditions under which systems operate. They allow us to use simpler models and equations while making predictions about the system's behavior. Since they are theoretically infinitely slow, they ensure that the system adjusts and remains in equilibrium with its surroundings, leading to predictable changes in state variables.
Examples & Analogies
Imagine slowly heating a pot of water on the stove while ensuring that the heat is evenly distributed. By doing it gradually, each molecule of water gets time to adjust to the heat, keeping the entire pot at a consistent temperature. This slow increase can be likened to a quasi-static process, ensuring that all changes are controlled and uniform.
Key Concepts
-
Quasi-static processes maintain equilibrium: These processes occur so slowly that the system is always in equilibrium with its surroundings.
-
Differential pressure and temperature: In quasi-static processes, the differences in pressure and temperature are infinitesimally small.
-
Types of processes: Quasi-static processes can include isothermal, adiabatic, isochoric, and isobaric processes.
Examples & Applications
An ideal gas expanding slowly in a piston, keeping the temperature constant is an example of an isothermal quasi-static process.
Compressing a gas very slowly in an insulated cylinder represents an adiabatic quasi-static process.
Memory Aids
Interactive tools to help you remember key concepts
Rhymes
If it’s slow and steady, we’re in the flow, Quasi-static, equilibrium, watch it grow!
Stories
In a calm lake, a boat sails slowly, adjusting to the gentle ripples, representing a quasi-static process — ever in balance, never rushing.
Memory Tools
Remember the acronym SIPE for Quasi-static processes: S - Slow, I - Infinitesimal, P - Pressure equilibrium, E - Energy exchange.
Acronyms
QUASI = Quick Understanding of An Infinitely slow process.
Flash Cards
Glossary
- Quasistatic Process
A thermodynamic process that occurs infinitely slowly to ensure the system remains in thermal and mechanical equilibrium with its surroundings.
- Thermal Equilibrium
The state of a system where temperature is uniformly distributed and does not change over time.
- Mechanical Equilibrium
The state of a system where the net external forces acting on it are zero.
- Isothermal Process
A process in which temperature of the system remains constant.
- Adiabatic Process
A process in which no heat is exchanged with the surroundings.
- Isochoric Process
A process where the volume of the system remains constant.
- Isobaric Process
A process where the pressure of the system remains constant.
Reference links
Supplementary resources to enhance your learning experience.
