REVERSIBLE AND IRREVERSIBLE PROCESSES
Enroll to start learning
You’ve not yet enrolled in this course. Please enroll for free to listen to audio lessons, classroom podcasts and take practice test.
Interactive Audio Lesson
Listen to a student-teacher conversation explaining the topic in a relatable way.
Introduction to Reversible Processes
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Today, we'll explore reversible processes. Can anyone tell me what a reversible process is?

Is it when a process can go back to its starting point without any changes?

Exactly! A reversible process means that both the system and surroundings can return to their original states without any other effects. Can anyone give me an example?

The isothermal expansion of a gas in a piston?

Correct! This process is idealized and occurs slowly, maintaining equilibrium at every stage. Remember the acronym 'QUIET' — Quasi-static, Uniform, Ideal, Equilibrium, and Temperature constant — to describe these conditions!

Does this mean all real processes are reversible?

No, in fact, most real-world processes are irreversible because they involve dissipative effects. Let's dive deeper into that next.

So, to recap, a reversible process is ideal and requires specific conditions like equilibrium. Keep 'QUIET' in mind!
Understanding Irreversible Processes
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Now, let’s discuss irreversible processes. Why do you think most processes in nature are irreversible?

Because they involve things like friction and heat loss?

Exactly! Irreversible processes arise mainly from two causes: processes that take systems to non-equilibrium states and the presence of dissipative effects. Can anyone think of an example of an irreversible process?

The combustion of fuel?

Great example! Once fuel combusts, you cannot revert the system to its original state. Also, think of the diffusion of cooking gas — it spreads and wouldn't spontaneously return to the cylinder.

So, all the natural processes we see are actually irreversible?

Yes! It's a rule, not an exception. Remember, irreversible processes illustrate the Second Law of Thermodynamics — they increase overall entropy!

To summarize, irreversible processes are governed by factors like friction and diffusion, which prevent complete reversibility.
The Second Law of Thermodynamics
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Let's connect our discussion to the Second Law of Thermodynamics. How does this law relate to reversible and irreversible processes?

It says that you can't have a perfect heat engine, right?

Correct! The Second Law states that no engine can convert all heat energy into work perfectly. Reversible processes serve as an ideal for efficiency but are not achievable. Why do you think this is important?

It helps us understand the limits of energy conversion.

Exactly, the highest efficiency for any heat engine is determined by reversible processes. In practice, all engines are at a loss due to irreversibility.

So, irreversible processes ultimately limit technological advancements?

Yes! That’s why understanding these concepts is vital. To wrap up, the Second Law highlights the inherent inefficiency in energy conversion due to irreversibility. Keep that in mind!
Introduction & Overview
Read summaries of the section's main ideas at different levels of detail.
Quick Overview
Standard
The section discusses the definitions of reversible and irreversible processes, highlighting the significance of these concepts in thermodynamics, particularly regarding their implications for work and heat. It explains that while reversible processes are idealized and quasi-static, irreversible processes commonly occur in nature due to factors like dissipative effects and the Second Law of Thermodynamics.
Detailed
REVERSIBLE AND IRREVERSIBLE PROCESSES
This section delves into the essential concepts of reversible and irreversible processes in thermodynamics. A thermodynamic process is deemed reversible if it allows both the system and its surroundings to return to their original states without affecting anything else in the universe. In contrast, irreversible processes are commonplace in nature and usually occur due to various reasons such as non-equilibrium states or dissipative effects like friction. The text stresses that most real-world phenomena are irreversible, in line with the Second Law of Thermodynamics, which stipulates that perfect efficiency in energy conversion is unattainable. Through examples including the heating of a vessel, diffusion of gases, and various mechanical processes, the distinction between these two types of processes is made clear, emphasizing that reversible processes serve as a theoretical benchmark for maximizing efficiency in thermodynamic systems.
Youtube Videos








Audio Book
Dive deep into the subject with an immersive audiobook experience.
Irreversibility in Nature
Chapter 1 of 4
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Imagine some process in which a thermodynamic system goes from an initial state i to a final state f. During the process the system absorbs heat Q from the surroundings and performs work W on it. Can we reverse this process and bring both the system and surroundings to their initial states with no other effect anywhere? Experience suggests that for most processes in nature this is not possible. The spontaneous processes of nature are irreversible. Several examples can be cited. The base of a vessel on an oven is hotter than its other parts. When the vessel is removed, heat is transferred from the base to the other parts, bringing the vessel to a uniform temperature (which in due course cools to the temperature of the surroundings). The process cannot be reversed; a part of the vessel will not get cooler spontaneously and warm up the base. It will violate the Second Law of Thermodynamics, if it did. The free expansion of a gas is irreversible. The combustion reaction of a mixture of petrol and air ignited by a spark cannot be reversed. Cooking gas leaking from a gas cylinder in the kitchen diffuses to the entire room. The diffusion process will not spontaneously reverse and bring the gas back to the cylinder. The stirring of a liquid in thermal contact with a reservoir will convert the work done into heat, increasing the internal energy of the reservoir. The process cannot be reversed exactly; otherwise, it would amount to conversion of heat entirely into work, violating the Second Law of Thermodynamics. Irreversibility is a rule rather than an exception in nature.
Detailed Explanation
Processes in nature are often one-way, meaning that once they occur, they cannot simply be reversed without additional changes occurring elsewhere. For example, when you heat a pot, the heat distributes, and once the pot cools down, you cannot revert the heat back to just the base without external work being done. This is a manifestation of the Second Law of Thermodynamics, which tells us that energy transformations are not always efficient, and some energy is always lost as waste.
Examples & Analogies
Think of a broken egg; once it's cracked and spilled, you can't simply put it back together and have it look exactly like it used to. Similarly, heat balancing in a vessel occurs spontaneously, but you can't make the cooler parts of the vessel heat back up without external input.
Causes of Irreversibility
Chapter 2 of 4
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Irreversibility arises mainly from two causes: one, many processes (like a free expansion or an explosive chemical reaction) take the system to non-equilibrium states; two, most processes involve friction, viscosity and other dissipative effects (e.g., a moving body coming to a stop and losing its mechanical energy as heat to the floor and the body; a rotating blade in a liquid coming to a stop due to viscosity and losing its mechanical energy with corresponding gain in the internal energy of the liquid). Since dissipative effects are present everywhere and can be minimised but not fully eliminated, most processes that we deal with are irreversible.
Detailed Explanation
The reasons why many processes are irreversible boil down to two main factors: reaching non-equilibrium (where the system cannot return to its original state without outside help) and the physical effects of friction and viscosity. For example, when you slide your hand across a table, the friction generates heat, and that energy is dispersed. You can't just clean it up; it gets lost to the environment, illustrating that not all processes can be perfectly reversed.
Examples & Analogies
Consider a car that comes to a stop because of friction with the road. The kinetic energy of the car is lost to heat—it's transformed into a less useful form which can't simply be recovered to propel the car back into motion without expending more energy.
Definition of Reversible Process
Chapter 3 of 4
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
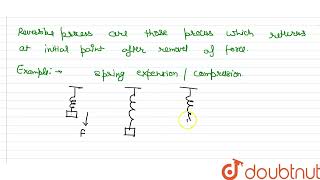
A thermodynamic process (state i → state f) is reversible if the process can be turned back such that both the system and the surroundings return to their original states, with no other change anywhere else in the universe. From the preceding discussion, a reversible process is an idealised notion. A process is reversible only if it is quasi-static (system in equilibrium with the surroundings at every stage) and there are no dissipative effects. For example, a quasi-static isothermal expansion of an ideal gas in a cylinder fitted with a frictionless movable piston is a reversible process.
Detailed Explanation
In theoretical terms, a reversible process is one where both the system and its surroundings can return to their original states without any energy loss or other changes happening in the universe. This hypothetical concept allows scientists to analyze energy efficiency and processes as if they were perfectly controllable, keeping in mind that real-world conditions often produce irreversible effects such as friction.
Examples & Analogies
Imagine a perfectly smooth surface where a ball rolls without any friction; if you were to lift it back to its original position, both the ball and the surface would be unchanged. This resembles the idealized reversible processes thermodynamics uses to set a standard for maximum efficiency in energy exchanges.
Importance of Reversibility in Thermodynamics
Chapter 4 of 4
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Why is reversibility such a basic concept in thermodynamics? As we have seen, one of the concerns of thermodynamics is the efficiency with which heat can be converted into work. The Second Law of Thermodynamics rules out the possibility of a perfect heat engine with 100% efficiency. But what is the highest efficiency possible for a heat engine working between two reservoirs at temperatures T1 and T2? It turns out that a heat engine based on idealized reversible processes achieves the highest efficiency possible. All other engines involving irreversibility in any way (as would be the case for practical engines) have lower than this limiting efficiency.
Detailed Explanation
Reversibility is essential in thermodynamics because it establishes the maximum efficiency boundaries of processes, specifically heat engines. These idealized models provide key insights into understanding how heat can be transformed into work while minimizing energy loss, thus setting standards against which actual engines can be evaluated.
Examples & Analogies
Consider a dancer performing a perfect routine on stage — every move is fluid and precise without any mishaps or errors. This represents an ideal reversible process. In contrast, a dancer who forgets steps or stumbles represents the irreversible processes found in most real-life situations. Perfect performances are rare, just like reversible processes in thermodynamics.
Key Concepts
-
Reversible Processes: Ideal processes that can return to their original states without any effects.
-
Irreversible Processes: Common processes that cannot revert without changes occurring.
-
Second Law of Thermodynamics: States the inefficiency of energy conversions in irreversible processes.
-
Quasi-static Processes: Ideal processes that occur slowly to maintain equilibrium.
-
Dissipative Effects: Causes of energy loss in real-world processes.
Examples & Applications
Heating of a pot, where heat transfer occurs from the base to the rest of the pot, leading to uniform temperature.
Gas diffusion in a room after a leak from a gas cylinder, which cannot spontaneously return to the cylinder.
Memory Aids
Interactive tools to help you remember key concepts
Rhymes
If a process can go back without a trace, it's reversible — a perfect place!
Stories
Imagine a slow gas cloud expanding in a balloon. If it could come right back, it's reversible; if it spreads out, it’s forever known!
Memory Tools
Remember 'QUIET' for reversible: Quasi-static, Uniform, Ideal, Equilibrium, Temperature constant.
Acronyms
D.E.S. for dissipative effects
Friction
Energy loss
Stopping forces.
Flash Cards
Glossary
- Reversible Process
A thermodynamic process that can return both the system and surroundings to their original states without any other effects.
- Irreversible Process
A process that cannot return a system and its surroundings to their original states without other changes occurring.
- Second Law of Thermodynamics
A law stating that energy conversions are never 100% efficient, and that irreversible processes tend to increase the overall entropy.
- Quasistatic Process
A process that occurs infinitely slowly, allowing the system to remain in equilibrium.
- Dissipative Effects
Energy losses in processes due to friction, viscosity, or other forms of resistance.
Reference links
Supplementary resources to enhance your learning experience.
