Definitions of Some Important Terms Pertaining to Coordination Compounds
Enroll to start learning
You’ve not yet enrolled in this course. Please enroll for free to listen to audio lessons, classroom podcasts and take practice test.
Interactive Audio Lesson
Listen to a student-teacher conversation explaining the topic in a relatable way.
Coordination Entity
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

A coordination entity consists of a central metal atom or ion bonded to a specific number of ions or molecules. For instance, the cobalt complex [CoCl3(NH3)3] shows cobalt at the center with ammonia and chloride ligands surrounding it.

So, the metal ion is the central part of the coordination entity?

Exactly! And we can think of it as the 'hub' around which the other ions or molecules revolve. Can anyone mention another example of a coordination entity?

How about [Ni(CO)4]?

Great example! Remember, each coordination entity is characterized by the geometry formed by these ligands around the central atom.
Central Atom/Ion
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

The central atom or ion is the focal point of a coordination entity to which ligands bond. Can anyone explain the significance of the central atom?

Is it like the 'heart' of the entity, determining its properties?

That's a fantastic way to think about it! The properties of the coordination complex greatly depend on the metal at the center. Each central atom also has an oxidation state that reflects its electron count. Why is understanding this oxidation state important?

It helps predict how the metal will react in different situations.

Precisely! Renowned metals like cobalt and nickel have specific coordination entities that show their reactivity.
Ligands
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Now, let’s discuss ligands. These can be ions or molecules that bond to the central atom. Can anyone differentiate between unidentate and polydentate ligands?

Unidentate ligands bind through one donor atom, whereas polydentate can bind through multiple donor atoms.

Correct! A good example of a hexadentate ligand is EDTA, which can bind through six donor atoms. What do you think are the benefits of having polydentate ligands?

I think they make the complexes more stable.

Exactly! These chelate complexes are often harder to break due to their multiple bonding points.
Coordination Number and Sphere
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Next, let’s discuss coordination number. What does the term 'coordination number' refer to?

It’s the number of donor atoms bonded to the central metal.

Exactly! For example, [PtCl6] has a coordination number of 6. Now, what is the coordination sphere?

That’s the group of ligands and central atom together, right?

Correct! And outside the coordination sphere are counter ions. This structure helps us understand how coordination compounds interact in solutions.
Coordination Polyhedron and Complexes
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Lastly, let’s discuss coordination polyhedra. What are the typical geometries of coordination complexes?

There’s octahedral, tetrahedral, and square planar.

Excellent! Each geometry corresponds to different coordination numbers and complexes like [Co(NH3)6] being octahedral. Knowing the geometry helps us predict reactivity and properties.

And what about homoleptic and heteroleptic complexes?

Great question! Homoleptic complexes have one type of ligand, while heteroleptic complexes possess different types. Each affects the properties and stability of the complex.
Introduction & Overview
Read summaries of the section's main ideas at different levels of detail.
Quick Overview
Standard
The section provides detailed definitions of key terminology essential for understanding coordination compounds, such as coordination entities, central metal ions, ligands, and coordination numbers, and explains the significance of these concepts in the context of chemistry.
Detailed
Definitions of Some Important Terms Pertaining to Coordination Compounds
In this section, we explore crucial terminology used in the field of coordination chemistry. The coordination entity consists of a central metal atom or ion surrounded by a specific number of ions or molecules known as ligands. The central atom/ion is the hub of this entity, where ligands attach in a characteristic geometrical arrangement, termed as coordination number (CN).
Ligands can vary in size and the number of donor atoms they utilize to bond to the metal. Based on their bonding characteristics, ligands are categorized as unidentate, didentate, or polydentate. The coordination number reflects how many donor atoms are bonded to the metal and can inform on geometric arrangement, such as octahedral, tetrahedral, or square planar. The coordination sphere includes the central atom and the attached ligands, while counter ions denote the ions not bonded directly to the metal. This section enhances our understanding of the complexity and significance of coordination compounds in chemistry by laying out these foundational terms.
Youtube Videos







Audio Book
Dive deep into the subject with an immersive audiobook experience.
Coordination Entity
Chapter 1 of 8
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
A coordination entity constitutes a central metal atom or ion bonded to a fixed number of ions or molecules. For example, [CoCl3(NH3)3] is a coordination entity in which the cobalt ion is surrounded by three ammonia molecules and three chloride ions. Other examples are [Ni(CO)4], [PtCl2(NH3)2], [Fe(CN)6], [Co(NH3)6].
Detailed Explanation
A coordination entity is the main structure in coordination chemistry. It consists of a central metal atom or ion that is surrounded by a specific number of ions or molecules, collectively known as ligands. For instance, in the coordination entity [CoCl3(NH3)3], cobalt (Co) is surrounded by three chloride ions (Cl) and three ammonia molecules (NH3). Each of these ligands is attached to the cobalt atom via a bond that involves coordinating the electrons, where the ligands share pairs of electrons with the central atom.
Examples & Analogies
Imagine the central metal atom like the manager of a restaurant and the ligands as the staff. The manager (cobalt) needs specific staff (three ammonia and three chloride ions) to effectively run the restaurant (the coordination entity). Just as the manager works with this fixed team, the metal center can only bond with a certain number of ligands based on its coordination number.
Central Atom/Ion
Chapter 2 of 8
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
In a coordination entity, the atom/ion to which a fixed number of ions/groups are bound in a definite geometrical arrangement around it, is called the central atom or ion. For example, the central atom/ion in the coordination entities: [NiCl2(H2O)4], [CoCl(NH3)5], and [Fe(CN)6] are Ni, Co, and Fe, respectively. These central atoms/ions are also referred to as Lewis acids.
Detailed Explanation
The central atom or ion is the core of the coordination entity around which ligands are arranged in a specific geometrical pattern. This atom typically belongs to transition metals, and due to their ability to accept electron pairs, they are classified as Lewis acids. In the examples provided, nickel (Ni) is the central atom in [NiCl2(H2O)4], cobalt (Co) in [CoCl(NH3)5], and iron (Fe) in [Fe(CN)6]. The geometrical arrangement affects the properties and reactivity of the coordination complex significantly.
Examples & Analogies
Think of the central atom as a hub in a wheel, with spokes extending outwards representing the ligands attached to it. Just like in a bicycle wheel, changing the hub or the way spokes are aligned affects how the wheel performs and its overall structure. Similarly, when ligands bind to a central metal ion, they define the shape and stability of the complex.
Ligands
Chapter 3 of 8
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
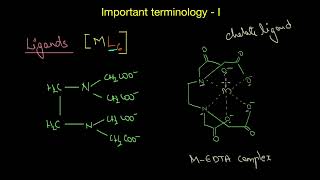
The ions or molecules bound to the central atom/ion in the coordination entity are called ligands. These may be simple ions such as Cl–, small molecules such as H2O or NH3, larger molecules such as H2NCH2CH2NH2 or N(CH2CH2NH2)3, or even macromolecules like proteins. Ligands may be classified based on the number of donor atoms they possess.
Detailed Explanation
Ligands are a vital part of coordination compounds as they donate electron pairs to the central atom, forming coordination bonds. They can be classified based on how many atoms they use to bind. For example, unidentate ligands like Cl– or NH3 bind through one donor atom, while didentate ligands, like ethylenediamine (H2NCH2CH2NH2), can bind at two points. Polydentate ligands bind at multiple sites, with EDTA being a classic example that can form up to six bonds to a central metal atom.
Examples & Analogies
You can think of ligands as keys that fit into a lock (the central atom). A key that opens a door at one point represents an unidentate ligand, whereas a key that can ensure a tighter fit at two points would symbolize a didentate ligand. The tighter and more effective the bond (like having multiple keys for a single lock), the more stable the coordination compound.
Coordination Number
Chapter 4 of 8
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
The coordination number (CN) of a metal ion in a complex can be defined as the number of ligand donor atoms to which the metal is directly bonded. For example, in the complex ions, [PtCl6]2– and [Ni(NH3)4], the coordination number of Pt and Ni are 6 and 4 respectively.
Detailed Explanation
The coordination number indicates how many ligands are bonded to a central metal ion directly through their donor atoms. This concept helps in understanding the geometry of the coordination compound. For instance, in [PtCl6]2–, the platinum ion is bonded to six chlorine ions, leading to an octahedral shape, while in [Ni(NH3)4], the nickel ion is bonded to four ammonia molecules, resulting in a tetrahedral arrangement.
Examples & Analogies
Visualize the coordination number as seating arrangements around a dinner table. If there are six guests (ligands) at a round table (central metal), it forms a larger, fully occupied arrangement (octahedral). Conversely, four guests arranged in a smaller square table setup (tetrahedral) would illustrate a lower coordination number. Just like in a dinner arrangement, the number of guests determines how the table is set.
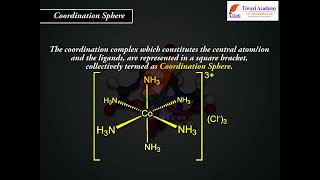
Coordination Sphere
Chapter 5 of 8
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
The central atom/ion and the ligands attached to it are enclosed in square brackets and are collectively termed as the coordination sphere. The ionisable groups are written outside the bracket and are called counter ions. For example, in the complex K4[Fe(CN)6], the coordination sphere is [Fe(CN)6]4– and the counter ion is K+.
Detailed Explanation
The coordination sphere is a crucial concept in determining the structure of coordination compounds. It is defined as the combination of the central metal atom and its attached ligands, typically represented inside square brackets. The ions outside this bracket do not interact directly with the metal-ligand bonds but balance the charge of the coordination sphere. In K4[Fe(CN)6], the complex carries a charge of -4, balanced by four potassium ions (K+) outside the brackets.
Examples & Analogies
Think of the coordination sphere as a protective case holding a delicate item (the complex). The item inside (the central atom and ligands) is secure in its casing, while other items (counter ions) placed around it help maintain the overall structure without interfering with the inner workings. It’s like a high-tech drone secured in its carrying case, needing its surroundings to remain safe.
Coordination Polyhedron
Chapter 6 of 8
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
The spatial arrangement of the ligand atoms which are directly attached to the central atom/ion defines a coordination polyhedron about the central atom. The most common coordination polyhedra are octahedral, square planar, and tetrahedral.
Detailed Explanation
The coordination polyhedron gives insight into how ligands are arranged around the central atom. The shape created by these ligand positions helps define the overall geometry of the coordination compound. Common structures include octahedral (six ligands arranged around the central atom), square planar (four ligands arrange in a square around the atom), and tetrahedral (four ligands diverging from the central atom). Each shape has unique properties and reactivity patterns based on its geometry.
Examples & Analogies
Visualizing the coordination polyhedron is similar to arranging students in a classroom for a group photo. In an octahedral arrangement, students stand in two rows of three around a central figure. In a square planar configuration, students form a square, while in a tetrahedral layout, they spread out from the center to form a triangular pyramid shape. The way they are arranged affects how the picture will turn out, just as the coordination shape affects how the compound behaves.
Oxidation Number of Central Atom
Chapter 7 of 8
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
The oxidation number of the central atom in a complex is defined as the charge it would carry if all the ligands were removed along with the electron pairs that are shared with the central atom. The oxidation number is represented by a Roman numeral in parenthesis following the name of the coordination entity.
Detailed Explanation
The oxidation number indicates the hypothetical charge of the central metal atom if all ligands were treated as completely ionic (derecognizing shared electron pairs). This concept is crucial for understanding the oxidation state of the metal, which impacts its reactivity and role in chemical reactions. For example, in [Cu(CN)4], copper has an oxidation number of +1, denoted as Cu(I) in its nomenclature.
Examples & Analogies
Think of the oxidation number as the credit score of a person in a financial institution. Just as a higher score indicates better credit worthiness (which affects borrowing power), a higher oxidation number shows how reactive an atom can be in forming compounds. Removing the ligands can give a clearer picture of the entity's charge and activity potential, just as evaluating credit reveals a person's financial credibility.
Homoleptic and Heteroleptic Complexes
Chapter 8 of 8
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Complexes in which a metal is bound to only one kind of donor groups, e.g., [Co(NH3)6]3+, are known as homoleptic. Complexes in which a metal is bound to more than one kind of donor groups, e.g., [Co(NH3)4Cl2]2+, are known as heteroleptic.
Detailed Explanation
The classification of complexes into homoleptic and heteroleptic is essential in coordination chemistry. Homoleptic complexes consist exclusively of one type of ligand bound to the metal, such as [Co(NH3)6]3+, where only ammonia molecules act as ligands. Conversely, heteroleptic complexes contain different types of ligands, exemplified by [Co(NH3)4Cl2]2+, which bonds both ammonia and chlorine ligands to the metal. This categorization affects the chemical behavior and potential applications of the complexes.
Examples & Analogies
Consider a homoleptic complex like a single-flavored ice cream cone, where every scoop is vanilla (only one type of ligand). In contrast, a heteroleptic complex is like a sundae with different flavors, including chocolate and strawberry (multiple types of ligands). Each type uniquely determines the taste and overall experience, paralleling how various ligands influence a compound's characteristics.
Key Concepts
-
Coordination Entity: A combination of a metal atom and the ligands bonded to it.
-
Central Atom: The central metal atom around which ligands are arranged.
-
Ligands: Ions or molecules attached to the central atom, classified by their donor atoms.
-
Coordination Number: The total number of bonds formed between ligands and the central metal atom.
-
Coordination Sphere: The enclosed structure of the central atom and its ligands.
-
Coordination Polyhedron: The geometrical arrangement of ligands around the central atom.
Examples & Applications
The complex [Ni(CO)4] illustrates a coordination entity with nickel as the central metal atom and carbon monoxide as a ligand.
In the complex [Fe(CN)6]4-, iron serves as the central atom with cyano ligands, demonstrating a coordination number of 6.
Memory Aids
Interactive tools to help you remember key concepts
Rhymes
Coordination is where metal meets, with ligands binding in structured beats.
Stories
Imagine a central king (metal) surrounded by knights (ligands) defending their territory (coordination sphere) in various formations (polyhedra).
Memory Tools
For the types of ligands: UNI-dentate, DI-dentate, POLY-dentate, remember: 'One for the road, two for the show, and many for the glow!'
Acronyms
SCOPE
Sphere
Central atom
Oxidation number
Polyhedron
Entity - key aspects of coordination compounds.
Flash Cards
Glossary
- Coordination Entity
A central metal atom or ion bonded to a definite number of ligands.
- Central Atom/Ion
The atom or ion to which ligands are bonded in a coordination entity.
- Ligands
Ions or molecules bound to the central atom in a coordination entity.
- Coordination Number (CN)
The number of ligand donor atoms bonded to the central metal atom or ion.
- Coordination Sphere
The central metal and the ligands attached to it, enclosed in square brackets.
- Coordination Polyhedron
The spatial arrangement of ligand atoms around the central atom.
- Oxidation Number
The charge of the central atom if all shared electrons with ligands are removed.
- Homoleptic Complex
A complex with a single type of ligand bound to the metal.
- Heteroleptic Complex
A complex with more than one type of ligand bound to the metal.
Reference links
Supplementary resources to enhance your learning experience.
