Nomenclature of Coordination Compounds
Enroll to start learning
You’ve not yet enrolled in this course. Please enroll for free to listen to audio lessons, classroom podcasts and take practice test.
Interactive Audio Lesson
Listen to a student-teacher conversation explaining the topic in a relatable way.
Introduction to Nomenclature
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Today, we'll start our exploration of nomenclature in coordination compounds. Can anyone tell me why proper naming is vital in chemistry?

I think it helps in avoiding confusion with different compounds.

Exactly! Proper nomenclature ensures we communicate clearly about compounds, especially when dealing with isomers. Now, what do you think makes naming coordination compounds unique?

Is it because they have both a central metal and ligands?

That's right! Coordination compounds consist of a central metal atom bonded to ligands. This brings us to how we formulate their names and write their chemical formulas.
Writing Formulas
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

When writing formulas, the central metal is listed first. What comes next?

The ligands in alphabetical order, regardless of their charges!

Exactly! We also enclose the entire coordination entity in square brackets. Now, can anyone give an example of what this looks like?

Like [Co(NH3)6]Cl3, where cobalt is first, followed by the ammonia ligands?

Great job! Remember to consider any charges and how they affect the formula, which we will discuss in our next session.
Naming Coordination Compounds
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Now that we understand how to write formulas, let’s talk about naming coordination compounds. What do you think is the first step?

Identifying the cation first?

Correct! The cation is named first, followed by ligands in alphabetical order. How do we change the names of anionic ligands?

They end in –ido, like chlorido for Cl-.

Yes! Plus, always remember that oxidation states in Roman numerals come after the name of the metal. Let's see examples of these names.
Examples of Naming
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Let’s work through naming examples, such as [Cr(NH3)3(H2O)3]Cl3. What's the systematic name?

It’s triamminetriaquachromium(III) chloride.

Correct! What about [Co(H2NCH2CH2NH2)3]2(SO4)3?

It would be tris(ethane-1,2-diamine)cobalt(III) sulfate.

Excellent work! Look how the systematic naming reflects the structure. Let's summarize key points on nomenclature.
Charge and Counter Ions in Naming
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Finally, we need to understand how charges play a role. What do you think happens with the counter ions?

They balance out the charge of the coordination entity, right?

Exactly! The overall charge of the compound should be neutral. So in K3[Fe(CN)6], the 3 potassium ions balance the -3 charge of the complex.

That makes sense! This helps in understanding how the overall compound is structured.

Great summary! Remember, understanding nomenclature is key in chemistry, especially for isomers.
Introduction & Overview
Read summaries of the section's main ideas at different levels of detail.
Quick Overview
Standard
In this section, we learned about the importance of nomenclature in coordination chemistry, defining key rules for naming and formulating mononuclear coordination compounds. It emphasizes the systematic approach to categorizing ligands, the central metal atom, and the overall structure, adhering to IUPAC guidelines.
Detailed
In coordination chemistry, the nomenclature of coordination compounds is crucial for ensuring clarity and uniformity in identifying various entities. Following the IUPAC recommendations, the rules for writing formulas and naming coordination entities are meticulously established. The central metal is listed first, followed by the ligands in alphabetical order, with special considerations for ionic and neutral ligands. The section details how the entire coordination sphere is represented within square brackets, alongside the indication of charges for coordination entities. Furthermore, the process of naming involves specific rules for ligands, metal oxidation states, and the coordination numbers, with illustrative examples to clarify the application of these rules. Proper nomenclature aids in avoiding ambiguities in chemical communication and contributes significantly to understanding isomerism and complex formation in coordination compounds.
Youtube Videos









Audio Book
Dive deep into the subject with an immersive audiobook experience.
Importance of Nomenclature in Coordination Chemistry
Chapter 1 of 4
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Nomenclature is important in Coordination Chemistry because of the need to have an unambiguous method of describing formulas and writing systematic names, particularly when dealing with isomers.
Detailed Explanation
In coordination chemistry, it's crucial to have a clear and systematic way to name compounds. This is because coordination compounds can have multiple forms called isomers, which can differ in structure and properties but share the same chemical formula. Clear nomenclature allows chemists to communicate effectively about these compounds without confusion.
Examples & Analogies
Think of naming coordination compounds like giving specific directions in a city. If you tell someone to meet you at 'the restaurant', they might get lost because there could be many restaurants. However, if you specify 'the Italian restaurant on Main Street,' there’s no confusion. In the same way, systematic naming helps chemists pinpoint exactly which molecule they are discussing.
Formulas of Mononuclear Coordination Entities
Chapter 2 of 4
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
The formula of a compound is a shorthand tool used to provide basic information about the constitution of the compound in a concise and convenient manner. Mononuclear coordination entities contain a single central metal atom. The following rules are applied while writing the formulas:
(i) The central atom is listed first.
(ii) The ligands are then listed in alphabetical order. The placement of a ligand in the list does not depend on its charge.
(iii) Polydentate ligands are also listed alphabetically. In case of abbreviated ligand, the first letter of the abbreviation is used to determine the position of the ligand in the alphabetical order.
(iv) The formula for the entire coordination entity, whether charged or not, is enclosed in square brackets. When ligands are polyatomic, their formulas are enclosed in parentheses. Ligand abbreviations are also enclosed in parentheses.
(v) There should be no space between the ligands and the metal within a coordination sphere.
(vi) When the formula of a charged coordination entity is to be written without that of the counter ion, the charge is indicated outside the square brackets as a right superscript with the number before the sign. For example, [Co(CN)6] , [Cr(H2O)6] , etc.
(vii) The charge of the cation(s) is balanced by the charge of the anion(s).
Detailed Explanation
Mononuclear coordination entities consist of one central metal atom bonded to various ligands. When writing the formula for these entities, certain rules must be followed. First, the central atom comes first in the formula. Next, ligands are listed in alphabetical order, regardless of their charge. Polydentate ligands (which can attach at multiple points) are treated similarly. The full formula is written in square brackets, with ligands in parentheses when necessary. It's essential to keep ligands directly attached to the central atom without spaces, and if the coordination entity has a charge, it's shown as a superscript.
Examples & Analogies
Writing chemical formulas can be likened to writing down a recipe. Just as you’d list ingredients in a specific order (like putting the main ingredient at the top), the formula for a coordination compound follows a similar order. If you said you had a dish with chicken and vegetables, you wouldn’t say 'vegetables, chicken' because it could confuse someone trying to make the dish. The clear format makes it easier for chemists to 'recreate' the compound in a lab.
Naming of Mononuclear Coordination Compounds
Chapter 3 of 4
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
The names of coordination compounds are derived by following the principles of additive nomenclature. Thus, the groups that surround the central atom must be identified in the name. They are listed as prefixes to the name of the central atom along with any appropriate multipliers.
The following rules are used when naming coordination compounds:
(i) The cation is named first in both positively and negatively charged coordination entities.
(ii) The ligands are named in an alphabetical order before the name of the central atom/ion. (This procedure is reversed from writing formula).
(iii) Names of the anionic ligands end in –o, those of neutral and cationic ligands are the same except aqua for H2O, ammine for NH3, carbonyl for CO and nitrosyl for NO. While writing the formula of coordination entity, these are enclosed in brackets ().
(iv) Prefixes mono, di, tri, etc., are used to indicate the number of the individual ligands in the coordination entity. When the names of the ligands include a numerical prefix, then the terms, bis, tris, tetrakis are used, the ligand to which they refer being placed in parentheses. For example, [NiCl2(PPh3)2] is named as dichloridobis(triphenylphosphine)nickel(II).
(v) Oxidation state of the metal in cation, anion or neutral coordination entity is indicated by Roman numeral in parenthesis.
(vi) If the complex ion is a cation, the metal is named the same as the element. For example, Co in a complex cation is called cobalt and Pt is called platinum. If the complex ion is an anion, the name of the metal ends with the suffix –ate. For example, Co in a complex anion, [Co(SCN)4] is called cobaltate. For some metals, the Latin names are used in the complex anions, e.g., ferrate for Fe.
(vii) The neutral complex molecule is named similar to that of the complex cation.
Detailed Explanation
Naming coordination compounds follows a structured approach to reduce ambiguity. The cation (the positively charged part) is named first, followed by the ligands listed alphabetically before the central atom. Anionic ligands are given specific endings (like -o) to distinguish them from cationic or neutral ligands, which keep their names. Each ligand can have multipliers prefixed to show how many are present, using terms like 'di-' for two. The oxidation number of the metal is also included in Roman numerals. For example, in the case of a cation, cobalt is simply called cobalt, while an anionic version changes the name to cobaltate.
Examples & Analogies
Consider this like naming a family. Just as the last name (like 'Smith') identifies a family, the central atom represents the main character in the coordination compound. The first names (the ligands) are arranged alphabetically to avoid confusion. If you have two siblings named 'Alice' and 'Bob,' you wouldn’t randomly refer to 'Bob' first and risk someone thinking of Alice instead. Each name aspect is tailored to keep things distinct and clear, which is the goal in naming coordination compounds.
Examples of Nomenclature
Chapter 4 of 4
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
The following examples illustrate the nomenclature for coordination compounds:
- [Cr(NH3)3(H2O)3]Cl3 is named as: triamminetriaquachromium(III) chloride
Explanation: The complex ion is inside the square bracket, which is a cation. The amine ligands are named before the aqua ligands according to alphabetical order. Since there are three chloride ions in the compound, the charge on the complex ion must be +3 (since the compound is electrically neutral). From the charge on the complex ion and the charge on the ligands, we can calculate the oxidation number of the metal. In this example, all the ligands are neutral molecules. Therefore, the oxidation number of chromium must be the same as the charge of the complex ion, +3.
- [Co(H2NCH2CH2NH2)3]2(SO4)3 is named as: tris(ethane-1,2-diamine)cobalt(III) sulphate
Explanation: The sulphate is the counter anion in this molecule. Since it takes 3 sulphates to bond with two complex cations, the charge on each complex cation must be +3. Further, ethane-1,2-diamine is a neutral molecule, so the oxidation number of cobalt in the complex ion must be +3. Remember that you never have to indicate the number of cations and anions in the name of an ionic compound.
- [Ag(NH3)2][Ag(CN)2] is named as: diamminesilver(I) dicyanidoargentate(I)
Detailed Explanation
Examples of naming coordination compounds illustrate how to apply the earlier rules in practice. Each example shows the systematic approach to consider the oxidations state, ligand names, and overall charges. The first example demonstrates how to calculate the charge and name ligands correctly. The other two provide varied cases, including cation-anion relationships and how they influence naming.
Examples & Analogies
Just like how you might reference friends in a system of titles (like 'Captain' in a group of friends), the names of coordination compounds give specific roles to each part of the compound—central metal ion, ligands, and charged complexes. The names become a structured system, ensuring everyone understands who holds what 'rank' in the formation.
Key Concepts
-
Nomenclature: A systematic method for naming coordination compounds.
-
Ligands: Molecules or ions that bind to a central metal atom, which can be unidentate or polydentate.
-
Coordination Sphere: The central metal atom and its affiliated ligands enclosed in square brackets, affecting charge representation.
Examples & Applications
Example 1: [Co(NH3)4Cl2] is named as 'tetrachloridobis(ammine)cobalt(II)' showing how prefixes and oxidation states are incorporated.
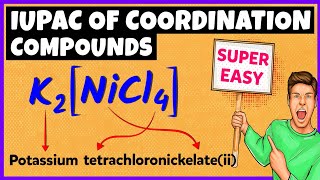
Example 2: K4[Fe(CN)6] is potassium hexacyanoferrate(II) showcasing the need for identifying counter ions.
Memory Aids
Interactive tools to help you remember key concepts
Rhymes
When naming coordination compounds, keep them in line, central first, then ligands, it will be just fine!
Stories
Imagine a central castle (the metal) surrounded by friendly neighbors (the ligands) who help it keep balance on the hill (the charges).
Memory Tools
CLAMP - Central atom, Ligands, Arrange alphabetically, Metal first, Place within brackets.
Acronyms
LANC - Ligand names alphabetically, Atom first, Number of ox state, Charge applies.
Flash Cards
Glossary
- Coordination Entity
A central metal atom or ion bonded to specific ions or neutral molecules in a fixed geometric arrangement.
- Central Atom/Ion
The atom or ion in a coordination entity to which ligands are bound.
- Ligand
An ion or molecule that binds to a metal atom/ion, serving as a donor atom for electron pairs.
- Coordination Number
The number of ligand donor atoms bonded directly to the central metal ion in a complex.
- Coordination Sphere
The central atom/ion and the ligands attached to it, represented within square brackets.
- Oxidation Number
The charge the central atom would have if all ligands were removed.
- Homoleptic Complex
A coordination compound containing only one type of ligand.
- Heteroleptic Complex
A coordination compound containing more than one type of ligand.
Reference links
Supplementary resources to enhance your learning experience.
