Limitations of Crystal Field Theory
Enroll to start learning
You’ve not yet enrolled in this course. Please enroll for free to listen to audio lessons, classroom podcasts and take practice test.
Interactive Audio Lesson
Listen to a student-teacher conversation explaining the topic in a relatable way.
Foundation of Crystal Field Theory
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Hello everyone! Today we are discussing the Crystal Field Theory. Can anyone tell me what CFT primarily focuses on?

It focuses on the interactions between metal ions and ligands.

Exactly! CFT treats these interactions as purely ionic. Now, while it accounts for structure and color, it has some limitations. Can anyone name what one of those limitations might be?

Maybe it doesn't account for covalent bonding?

Correct! It assumes all bonds are ionic, ignoring any covalent character. Remembering that helps in understanding why certain predictions fail.

So does that mean CFT is completely wrong?

Not quite; it provides a foundation, but we need to look beyond it for complex cases. Today’s take-home point is that while CFT is useful, its boundaries guide us to explore further models!
Limitations in Predictive Power
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Now, let's delve deeper into CFT's limitations. Why do you think it struggles to accurately predict magnetic properties?

Because it can't quantify magnetic moments effectively?

Exactly! CFT doesn't provide detailed insights into how ligands influence magnetic properties. Can anyone relate this to color prediction?

It probably fails to predict the colors of compounds accurately because of its oversimplification.

Absolutely! CFT fails to explain why certain complexes have specific colors. Remember the relationship between color and wavelength for a better grasp!
Crystal Field Theory vs. Modern Theories
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

As we discuss these limitations, let’s compare CFT to Ligand Field Theory. Why might LFT offer better predictions?

LFT includes covalent character in bonding, right?

Exactly! This inclusion allows for a comprehensive understanding of bonding interactions. Can anyone summarize why we should know both theories?

CFT helps with basics, but LFT gives a deeper understanding helping us predict and explain properties better.

Well put! Think of CFT as the stepping stone to more complex models. Each theory expands our understanding of coordination complexes.
Introduction & Overview
Read summaries of the section's main ideas at different levels of detail.
Quick Overview
Standard
While CFT successfully accounts for the formation, structures, colors, and magnetic properties of coordination compounds by treating ligands as point charges, it overlooks the covalent character in bonding and fails to accurately predict behaviors associated with varying ligand types, leading to discrepancies in its application.
Detailed
Limitations of Crystal Field Theory
Crystal Field Theory (CFT) provides a framework to understand bonding in coordination compounds by focusing on the interactions between metal ions and ligands. As an electrostatic model, it suggests that the metal-ligand bond is purely ionic, arising from electrostatic interactions.
Key Limitations:
- Assumptions About Ligands: CFT considers ligands as point charges, which oversimplifies real-world interactions. Consequently, anionic ligands, which theoretically exert the strongest splitting effects, are often observed at the bottom of the spectrochemical series, countering CFT predictions.
- Neglecting Covalent Interactions: CFT predominantly ignores the covalent character that can exist in metal-ligand bonding. This aspect is crucial in explaining the complex behaviors of transition metals in coordination environments.
- Magnetic and Spectroscopic Predictions: Though CFT explains certain magnetic and color properties adequately, it does not consistently provide quantitative interpretations of these characteristics. The theory does not account for spectral data effectively nor does it provide precise values for crystal field splitting energy (Δ), which varies significantly among different metal-ligand interactions.
- Directional Bonding: The theory does not explain why certain coordination compounds exhibit directional bonding properties. The spacial orientation of ligands can significantly impact coordination geometry, which CFT fails to incorporate.
- Complex Cases: In scenarios involving mixed ligand environments or complexes with multiple coordination modes, CFT becomes inadequate, as it struggles to account for differential ligand effects and structural changes.
In essence, while CFT serves as a valuable tool in coordination chemistry, its limitations necessitate the introduction of theories like Ligand Field Theory (LFT) and Molecular Orbital Theory (MOT) for a more comprehensive understanding.
Youtube Videos







Audio Book
Dive deep into the subject with an immersive audiobook experience.
Overview of Crystal Field Theory
Chapter 1 of 4
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
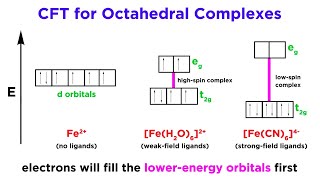
The crystal field model is successful in explaining the formation, structures, colour and magnetic properties of coordination compounds to a large extent.
Detailed Explanation
The crystal field theory (CFT) primarily explains how coordination compounds are formed and their structures. It does this by viewing ligands as point charges that create an electrostatic field around the central metal ion, influencing its behavior and properties. CFT helps elucidate the colors of coordination compounds and their magnetic properties.
Examples & Analogies
Think of CFT as a magnetic field around a planet, where the planet (the metal ion) feels the effect of the nearby stars (the ligands). These stars can affect how the planet spins (its properties) based on their positions.
Weakness of Point Charge Assumption
Chapter 2 of 4
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
However, from the assumptions that the ligands are point charges, it follows that anionic ligands should exert the greatest splitting effect.
Detailed Explanation
CFT assumes that ligands behave as point charges that exert forces solely based on electrostatics. According to this model, negatively charged (anionic) ligands should create a strong splitting of the d-orbitals in the central metal ion. However, empirical data shows that this is not always the case, which indicates that other effects are in play.
Examples & Analogies
Imagine a game where players (ligands) throw balls (negative charges) at a target (the metal ion). According to CFT, we would expect heavy balls (strong anionic ligands) to hit harder and move the target more. Yet, sometimes lighter balls (neutral or weaker ligands) cause more movement, showing that the initial assumption isn’t entirely accurate.
Neglect of Covalent Character
Chapter 3 of 4
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Further, it does not take into account the covalent character of bonding between the ligand and the central atom.
Detailed Explanation
CFT primarily focuses on electrostatic interactions and does not recognize that there is often a covalent component to the bonding between metal ions and ligands. This means that some bonding characteristics, like the angle and length of bonds, aren't fully explained by CFT alone.
Examples & Analogies
Imagine building a bridge where you only consider the weight of the cars (electrostatic forces) but ignore how the materials (covalent bonds) themselves interact and hold the bridge up. In reality, both aspects are crucial for understanding how the bridge functions.
Implications of Limitations
Chapter 4 of 4
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
These are some of the weaknesses of CFT, which are explained by ligand field theory (LFT) and molecular orbital theory which are beyond the scope of the present study.
Detailed Explanation
The limitations noted earlier suggest that while CFT provides a foundation for understanding coordination compounds, more sophisticated theories like ligand field theory (LFT) and molecular orbital theory (MOT) offer a more comprehensive explanation of bonding and properties, particularly for complexes featuring strong field ligands.
Examples & Analogies
Consider CFT like a basic map providing the outline of a city. While it shows you the main streets, it doesn't reveal the hidden paths or the details of buildings (like LFT and MOT) that give you a complete understanding of how to navigate the city effectively.
Key Concepts
-
CFT explains coordination compound properties.
-
CFT limitations include ignoring covalent bonding.
-
Magnetic properties predictions are inadequate.
-
The spectrochemical series ranks ligand effects.
Examples & Applications
In octahedral complexes, the d-orbitals split into two energy levels due to ligand presence, contradicting CFT predictions regarding ligand interactions.
Certain metal complexes show unexpected colors—a result not predicted by CFT, highlighting its limitations.
Memory Aids
Interactive tools to help you remember key concepts
Rhymes
CFT in the field, bonds not so grand, ionic they are, but more needs to be planned.
Memory Tools
Think of 'CFT': 'Can't Form True' because it ignores covalent reality.
Stories
Imagine a party: the metal ion treats ligands as uninvited guests, only seeing them as point figures—missing their real interactions at the dance floor!
Acronyms
CFT
Crystal Field Tension—it holds the bond tight but doesn’t see the dance!
Flash Cards
Glossary
- Crystal Field Theory (CFT)
An electrostatic model that describes the bonding in coordination compounds as purely ionic interactions between metal ions and ligands.
- Ligand Field Theory (LFT)
An extension of Crystal Field Theory that incorporates covalent character into metal-ligand bonding.
- Spectrochemical Series
A list that ranks ligands based on their ability to split d-orbital energies in coordination compounds.
- Magnetic Moment
A value that indicates the number of unpaired electrons in a coordination compound, influencing its magnetic properties.
Reference links
Supplementary resources to enhance your learning experience.
