Optical Isomerism
Enroll to start learning
You’ve not yet enrolled in this course. Please enroll for free to listen to audio lessons, classroom podcasts and take practice test.
Interactive Audio Lesson
Listen to a student-teacher conversation explaining the topic in a relatable way.
Introduction to Optical Isomerism
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Today, we’re exploring the fascinating concept of optical isomerism. Can anyone tell me what they understand by isomers?

Are isomers just different versions of the same compound?

Exactly! Isomers are compounds with the same molecular formula but different arrangements of atoms. Today, we’ll focus on a specific type—optical isomerism. These are molecules that are non-superimposable mirror images.

So like my left and right hands?

Exactly! They're perfect examples of chirality. These optical isomers are often referred to as enantiomers. Let’s remember the term ‘enantiomers’ as the two forms of a compound that exhibit this optical activity.
Chirality and Coordination Compounds
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Chirality is crucial in coordination chemistry, especially with certain ligands. Can anyone think of a ligand that might introduce chirality?

What about ethylenediamine? I think it can connect to the metal in different ways.

Great example! Ethylenediamine is a bidentate ligand and can indeed create chiral complexes. When it binds in a way that forms two different non-superimposable arrangements, we can see optical isomerism.

Does this mean that these isomers can have different properties?

Yes, that's correct! Each enantiomer can react differently, especially in biological systems. It’s important to remember the phrase: ‘one drug, two effects’ when thinking about enantiomers!
Examples of Optical Isomers in Coordination Chemistry
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Let’s look at some examples of optical isomers. Who can describe the coordination compound cis-[Co(en)3]3+?

Isn’t that the one where different arrangements of ethylenediamine create two non-superimposable shapes?

Absolutely! This example shows the significance of arrangement. Can someone explain why we care about optical isomerism in drug design?

Different enantiomers might have different biological effects? Like one could be effective while the other is harmful.

Right on! The concept of 'chirality' in drugs is vital. Remember how this may affect interactions within our body!
Review and Summary
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

To wrap up, can someone summarize what optical isomerism is?

It's a type of stereoisomerism where two compounds are non-superimposable mirror images.

Great! And can anyone recall why this is important in chemistry?

It can impact how drugs work and their effectiveness, right?

Exactly! Remember that understanding these concepts leads us to appreciate the complexity of chemical interactions in biological systems.
Introduction & Overview
Read summaries of the section's main ideas at different levels of detail.
Quick Overview
Standard
Optical isomerism arises in coordination compounds exhibiting chirality, leading to non-superimposable mirror images called enantiomers. This phenomenon is significant in biological systems and can affect the compound's interactions and functions, particularly in pharmaceuticals.
Detailed
Detailed Summary
Optical isomerism is a form of stereoisomerism that occurs when a molecule can exist in two non-superimposable mirror image forms known as enantiomers. These isomers are often chiral, meaning they cannot be overlapped onto their mirror images, similar to how left and right hands are distinct. Chiral centers in a compound typically arise from carbon atoms that are bonded to four different groups. In coordination chemistry, specific geometries, typically those with tetrahedral and octahedral coordination, enable this chiral character to emerge, especially with bidentate ligands such as ethylenediamine (en).
Examples of Chiral Coordination Compounds
- Cis-[Co(en)3]3+: This octahedral complex exhibits optical activity as it can exist in two distinct forms.
- [PtCl2(en)2]: This complex shows distinct optical isomers depending on how the bidentate ligands are arranged. Optical isomerism plays a crucial role in the biological activity of molecules, which is especially important for drug design and efficacy.
In practical applications, the differences in behaviors between enantiomers can have significant implications, from taste variations in food to the differential effects in pharmacology, where one enantiomer may be therapeutically beneficial while the other could cause undesired side effects.
Youtube Videos







Audio Book
Dive deep into the subject with an immersive audiobook experience.
Definition of Optical Isomerism
Chapter 1 of 3
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Optical isomers are mirror images that cannot be superimposed on one another. These are called as enantiomers. The molecules or ions that cannot be superimposed are called chiral.
Detailed Explanation
Optical isomerism occurs when two compounds have the same molecular formula and connectivity of atoms but differ in three-dimensional arrangement. These isomers are called enantiomers. A key characteristic of enantiomers is that they are chiral, meaning they have non-superimposable mirror images. For example, if one enantiomer is rotated to the left, the other will rotate to the right, hence the terms 'dextro' (d) for right and 'laevo' (l) for left.
Examples & Analogies
Think of your left and right hands. They are mirror images of each other and cannot be placed on top of one another perfectly. This is similar to how optical isomers work; each isomer has the same composition but different spatial arrangements, making them unique.
Optical Activity of Complexes
Chapter 2 of 3
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
The two forms are called dextro (d) and laevo (l) depending upon the direction they rotate the plane of polarised light in a polarimeter (d rotates to the right, l to the left).
Detailed Explanation
Optical activity is a property of chiral substances that allows them to rotate plane-polarized light. Dextrorotatory (d) enantiomers rotate light to the right (clockwise), while levorotatory (l) enantiomers rotate light to the left (counterclockwise). This ability to rotate light serves as a critical marker for identifying and differentiating between enantiomers in a laboratory setting.
Examples & Analogies
Consider wearing polarized sunglasses. When you look through them, you might notice that some light is dimmed or filtered out due to the lens’s orientation. Similarly, when chiral compounds interact with polarized light, they change its path. This spiraling effect is what chemists measure when studying optical isomers.
Cis-Isomer and Optical Activity
Chapter 3 of 3
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
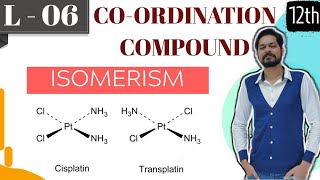
In a coordination entity of the type [PtCl2(en)2], only the cis-isomer shows optical activity.
Detailed Explanation
In coordination chemistry, the arrangement of ligands around a central metal can lead to optical isomerism. For certain complexes, such as the cis-isomer of [PtCl2(en)2], the spatial orientation allows for chirality, meaning these isomers have distinct optical properties. In contrast, the trans-isomer would have a symmetrical arrangement that does not exhibit optical activity because it can be superimposed on its mirror image.
Examples & Analogies
Imagine two people dressed identically, but one is standing with their arms crossed (the cis version) while the other has their arms at their sides (the trans version). The person with crossed arms may look different from their mirror image while the other does not, just like how the cis-isomer of [PtCl2(en)2] shows optical activity while the trans-isomer does not.
Key Concepts
-
Chirality: Non-superimposable mirror images of compounds.
-
Enantiomers: Optical isomers that exhibit different effects.
-
Bidentate Ligands: Ligands that can attach to a metal at two points, causing chirality.
Examples & Applications
Cis-[Co(en)3]3+: This octahedral complex exhibits optical activity as it can exist in two distinct forms.
[PtCl2(en)2]: This complex shows distinct optical isomers depending on how the bidentate ligands are arranged. Optical isomerism plays a crucial role in the biological activity of molecules, which is especially important for drug design and efficacy.
In practical applications, the differences in behaviors between enantiomers can have significant implications, from taste variations in food to the differential effects in pharmacology, where one enantiomer may be therapeutically beneficial while the other could cause undesired side effects.
Memory Aids
Interactive tools to help you remember key concepts
Rhymes
Enantiomers twirl and spin, mirror images locked from within.
Stories
Once upon a time, in a land of chemistry, two brothers named 'Chiral' lived. They looked the same but could never shake hands, showcasing how different they truly were, like mirrors without the overlap.
Memory Tools
C.H.I.R.A.L.: Compounds Having Identifiable Rigid Arrangement of Ligands.
Acronyms
C.A.R.E - Chirality Affects Reactions and Effects in biology.
Flash Cards
Glossary
- Optical Isomerism
A form of stereoisomerism whereby molecules exist as non-superimposable mirror images.
- Enantiomers
Pairs of optical isomers; compounds that are mirror images of each other.
- Chirality
The geometric property of a molecule having non-superimposable mirror images.
- Bidentate Ligand
A ligand that can bond to a metal atom at two distinct points.
Reference links
Supplementary resources to enhance your learning experience.
