Electrophilic aromatic substitution
Enroll to start learning
You’ve not yet enrolled in this course. Please enroll for free to listen to audio lessons, classroom podcasts and take practice test.
Interactive Audio Lesson
Listen to a student-teacher conversation explaining the topic in a relatable way.
Introduction to Electrophilic Aromatic Substitution
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Today, we're going to dive into Electrophilic Aromatic Substitution, or EAS. Can anyone tell me what they think this reaction involves?

Does it involve replacing hydrogen atoms in aromatic compounds with other groups?

Yes! And I think it has to do with electrophiles attacking something in benzene.

Correct! So, EAS specifically involves electrophiles attacking aromatic rings and substituting hydrogen atoms. This is crucial for building more complex aromatic compounds.

Why is it important in chemistry?

Great question! EAS allows chemists to modify aromatic compounds to create useful materials like drugs and dyes.

To remember this, think EAS: 'Electron Attack Substitution'—it highlights the importance of electron density in these reactions.

Remember, EAS reactions primarily happen at the ortho and para positions due to the electron-donating effects of groups like -OH in phenols.
Mechanisms of EAS
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Now let’s get into the mechanism of EAS. It proceeds in two main steps. Can someone describe the first step?

Isn’t it about forming a carbocation intermediate?

Exactly! The first step forms a sigma complex, where the benzene ring temporarily loses its aromaticity. Can anyone tell me what happens next?

After the carbocation forms, the last hydrogen is removed, right?

Yeah, and then the aromaticity returns!

Great! So the regeneration of aromaticity completes the reaction, and now we have an electrophile substituted for hydrogen. To memorize this, think of the acronym **C-R**, where 'C' stands for 'Carbocation' and 'R' for 'Return to aromaticity.'
Examples of EAS Reactions
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Let’s talk about some common reactions of EAS. One important example is the nitration of phenol. Can anyone explain what happens?

The phenol reacts with nitric acid to produce nitrophenols.

Exactly! Which positions do these nitrophenol isomers typically form?

They occur at the ortho and para positions!

Right! Another example is the halogenation of phenol, where bromine can add at ortho and para sites. This highlights EAS's versatility in creating diverse aromatic compounds. Can you all think of practical applications for these reactions?

Maybe in making dyes or pharmaceuticals?

Spot on! To recall these reactions, use the mnemonic 'Nectar Brings Sweet Electrophiles,' where N stands for Nitration and B for Bromination.
Introduction & Overview
Read summaries of the section's main ideas at different levels of detail.
Quick Overview
Standard
Electrophilic aromatic substitution is a fundamental reaction mechanism whereby aromatic compounds undergo substitution reactions with electrophiles. The presence of electron-donating groups, such as hydroxyl (-OH) in phenols, greatly facilitates these reactions by activating the aromatic ring and directing substitution towards ortho and para positions. This section explores the mechanisms, examples, and significance of EAS in synthetic organic chemistry.
Detailed
Electrophilic Aromatic Substitution (EAS)
Electrophilic aromatic substitution is a vital reaction mechanism in organic chemistry where electrophiles replace hydrogen atoms in an aromatic compound. This process is important for synthesizing various aromatic compounds and understanding electrophilic attack's nature on benzene systems.
Key Features of EAS
- Role of Activating Groups: The presence of activating groups such as -OH in phenols enhances the electron density of the aromatic ring, making it more susceptible to electrophilic attack. This interaction stabilizes carbocations formed during reactions, favoring substitution at ortho and para positions over the meta position.
- Electrophile: These can be various species, including nitronium ions (from nitration) or halogen cations (from halogenation).
- Mechanism: EAS typically involves several steps:
- Formation of a sigma complex (or arenium ion).
- Loss of a proton to regenerate the aromatic system.
Typical Reactions
- Halogenation: For example, phenol reacting with bromine yields brominated products primarily at ortho and para positions.
- Nitration: Phenols reacting with nitric acid produce a mixture of nitrophenol isomers.
Significance in Synthesis
Electrophilic aromatic substitution reactions are essential for synthesizing functionalized aromatic compounds, offering pathways to complex natural products and pharmaceutical agents. Understanding these reactions is crucial for chemists in both academic and industrial settings.
Youtube Videos








Audio Book
Dive deep into the subject with an immersive audiobook experience.
Understanding Electrophilic Aromatic Substitution
Chapter 1 of 3
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
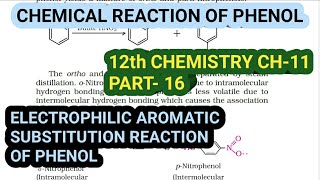
In phenols, the reactions that take place on the aromatic ring are electrophilic substitution reactions. The –OH group attached to the benzene ring activates it towards electrophilic substitution. Also, it directs the incoming group to ortho and para positions in the ring as these positions become electron-rich due to the resonance effect caused by –OH group.
Detailed Explanation
Electrophilic aromatic substitution is a type of reaction that occurs when an electrophile replaces a hydrogen atom on an aromatic ring. In phenols, the hydroxyl (-OH) group makes the ring more reactive because it increases the density of electrons in the aromatic system. As a result, this reaction commonly occurs at the ortho (adjacent) or para (across) positions relative to the –OH group. This is due to the resonance structures that can be stabilized by the –OH group.
Examples & Analogies
You can think of the aromatic ring as a party where the –OH group is the host making it more inviting for guests (electrophiles) to join and interact. Just like how a welcoming environment encourages guests to gather close, the resonance effect allows the electrophiles to easily approach and bond with the aromatic ring.
Nitration Reaction of Phenol
Chapter 2 of 3
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
The common electrophilic aromatic substitution reactions taking place in phenol are as follows: (i) Nitration: With dilute nitric acid at low temperature (298 K), phenol yields a mixture of ortho and para nitrophenols.
Detailed Explanation
When phenol undergoes nitration, it reacts with dilute nitric acid (HNO3) under controlled conditions. This results in a mixture of products: ortho-nitrophenol and para-nitrophenol. The ortho product forms faster because it is in closer proximity to the –OH group, while the para product is also favored because it is more stable. The separation of these isomers can be done through steam distillation due to their differing boiling points.
Examples & Analogies
Imagine you are making two types of fruit punch by adding lemon juice (phenol) to a mixture of water and sugar (nitration reaction). You can taste the punch immediately next to the lemon (ortho-nitrophenol) and the one further away (para-nitrophenol). Even though both recipes have lemon juice, the immediate one tastes different from the one a bit farther away since the flavors mix differently.
Halogenation of Phenol
Chapter 3 of 3
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Halogenation: On treating phenol with bromine, different reaction products are formed under different experimental conditions. (a) When the reaction is carried out in solvents of low polarity such as CHCl3 or CS2 and at low temperature, monobromophenols are formed. (b) When phenol is treated with bromine water, 2,4,6-tribromophenol is formed as a white precipitate.
Detailed Explanation
Halogenation involves the introduction of halogen atoms (like bromine) into the phenol molecule. Under specific conditions, like using non-polar solvents, phenol can undergo a more controlled reaction resulting in monobromophenols. However, if bromine water is used, this encourages multiple bromination leading to 2,4,6-tribromophenol, which precipitates out of the solution due to its limited solubility.
Examples & Analogies
Think of this like adding spices to a dish. If you sprinkle a little bit of salt (non-polar solvent), you'll get just the right amount of flavor. But if you pour a brine solution (bromine water), it overwhelms the dish and results in an entirely different taste, much like how adding too much leads to multiple bromine atoms being incorporated into the product.
Key Concepts
-
EAS: A fundamental mechanism for introducing electrophiles into aromatic compounds.
-
Activating Groups: Groups that increase the reactivity of aromatic rings towards electrophiles.
-
Ortho/Para Directing: The result of resonance stabilization that guides substitution preferentially to ortho and para positions.
Examples & Applications
The nitration of phenol yields 2-nitrophenol and 4-nitrophenol as products.
The bromination of phenol at low temperatures yields ortho-bromophenol and para-bromophenol.
Memory Aids
Interactive tools to help you remember key concepts
Rhymes
Electrophiles fly to rings with glee, substituting hydrogens, oh can’t you see!
Stories
Imagine a crowded party (the aromatic ring) where guests (hydrogens) are replaced by newcomers (electrophiles) for a more lively atmosphere.
Memory Tools
Remember O-P-E for 'Ortho-Para-Electrophilic,' highlighting the primary positions affected in EAS.
Acronyms
EAS
'Electron Attacks Substitution' to recall its requirements.
Flash Cards
Glossary
- Electrophile
A species that seeks electron-rich areas in molecules and can accept a pair of electrons.
- Sigma complex
An intermediate in electrophilic aromatic substitution where the aromaticity of the benzene ring is temporarily lost.
- Aromaticity
The stability and unique properties of cyclic compounds with delocalized conjugated pi-electron systems that follow Huckel's rule.
- Substitution reaction
A type of reaction where one atom or group is replaced by another atom or group.
Reference links
Supplementary resources to enhance your learning experience.
