Reaction with phosphorus trihalides
Enroll to start learning
You’ve not yet enrolled in this course. Please enroll for free to listen to audio lessons, classroom podcasts and take practice test.
Interactive Audio Lesson
Listen to a student-teacher conversation explaining the topic in a relatable way.
Introduction to Alcohol Reactions
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Today, we will discuss the reaction of alcohol with phosphorus trihalides. Can anyone tell me what we understand by phosphorus trihalides?

I think they are compounds like PCl3, PBr3, and PI3, which contain phosphorus and halogens.

Excellent! These compounds are commonly used to convert alcohols into alkyl halides. The hydroxyl group is replaced by a halogen.

So, how does this replacement actually happen in the reaction?
Mechanism of Alcohol to Alkyl Halide Conversion
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Let's delve into the mechanism now. The first step involves the formation of an alkoxide ion.

What do we mean by alkoxide ion?

Good question! An alkoxide ion is formed when the -OH group in alcohol gets replaced, facilitating the nucleophilic attack by halides.

Do these reactions happen quickly? How do we ensure they proceed to form the halide?

The reaction usually proceeds effectively due to lower activation energy provided by phosphorus trihalides. The intermediates formed help to push the reaction forward.
Practical Applications of the Reaction
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Why do you think we often convert alcohols to alkyl halides? What are some applications?

I suppose we use alkyl halides in many reactions for synthesis of other compounds, right?

Yes, like in the synthesis of ethers and other organic molecules!

Exactly! Alkyl halides are versatile intermediates in organic chemistry, helping to extend synthetic pathways effectively.
Introduction & Overview
Read summaries of the section's main ideas at different levels of detail.
Quick Overview
Standard
In this section, we learn about the reaction of alcohols with phosphorus trihalides, which leads to the formation of alkyl halides. The process is significant for understanding nucleophilic substitution reactions. The mechanism of this conversion is explored to reveal how alcohols lose their hydroxyl group and form halides effectively.
Detailed
The reaction involving alcohols with phosphorus trihalides (PCl3, PBr3, or PI3) offers an efficient method for converting alcohols into their corresponding alkyl halides. This process is particularly useful in organic synthesis where the -OH group of alcohols is substituted by a halogen. The mechanism of the reaction includes the formation of intermediate alkoxides that are subsequently transformed into alkyl halides. Plus, the use of phosphorus trihalides lowers the activation energy needed to facilitate the substitution reaction, which is essential in practical laboratory setups.
Youtube Videos









Audio Book
Dive deep into the subject with an immersive audiobook experience.
Overview of Reactions with Phosphorus Trihalides
Chapter 1 of 3
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Alcohols are converted to alkyl bromides by reaction with phosphorus tribromide.
Detailed Explanation
Phosphorus trihalides, specifically phosphorus tribromide (PBr3), are reagents used to convert alcohols into alkyl bromides. In this reaction, the hydroxyl group (-OH) of the alcohol gets replaced by a bromine atom, resulting in the formation of an alkyl bromide. This conversion is particularly valuable because it allows for the transformation of alcohols, which are often less reactive, into alkyl bromides that can participate in various substitution and elimination reactions.
Examples & Analogies
You can think of this reaction like swapping an old appliance in your home for a new, more efficient one. Just like an old refrigerator being replaced with a new model that works better and serves your needs, here, the alcohol is replaced by a more reactive alkyl bromide that can take part in further chemical reactions more effectively.
Mechanism of the Reaction with Phosphorus Trihalides
Chapter 2 of 3
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
The mechanism involves the formation of an alkoxyphosphonium intermediate followed by elimination to give the alkyl bromide.
Detailed Explanation
In the mechanism of this reaction, when an alcohol reacts with phosphorus tribromide, the first step involves the alcohol forming an alkoxyphosphonium intermediate. In this step, the -OH group of the alcohol attacks phosphorus, resulting in the displacement of a bromide ion. This intermediate can then lose a bromide ion, resulting in the formation of the desired alkyl bromide. The overall reaction efficiently replaces the -OH group with a bromine atom.
Examples & Analogies
Imagine you’re changing a car's tire. First, you use a wrench to loosen the bolts (similar to the formation of the intermediate), and once they're loose, you can easily remove the flat tire and replace it with a new one (which represents the final alkyl bromide). The tools used to assist in this task represent the roll of phosphorus tribromide in facilitating the replacement of the hydroxyl group.
Types of Alcohols and Their Reactivity in This Reaction
Chapter 3 of 3
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Primary, secondary, and tertiary alcohols behave differently with phosphorus trihalides. Generally, primary alcohols react more efficiently compared to tertiary alcohols.
Detailed Explanation
The reactivity of alcohols in reactions with phosphorus trihalides varies based on their structure. Primary alcohols typically react more readily because they can easily form stable intermediates that lead to the formation of the alkyl bromide. Secondary alcohols react at moderate speeds, while tertiary alcohols can sometimes form unwanted products due to steric hindrance, which may complicate or hinder the reaction process.
Examples & Analogies
Consider a crowded hallway in a school. If students (representing different types of alcohols) are trying to exit the hallway, primary alcohols have the easiest time getting out quickly, representing their efficiency in reactions. Secondary alcohols can make it through with a little more time, whereas tertiary alcohols might get stuck or delayed because of their bulky structure, making it harder for them to 'get through' the reaction.
Key Concepts
-
Phosphorus Trihalides: Used to convert alcohols to alkyl halides.
-
Mechanism Details: Involves formation of alkoxide intermediates.
-
Practical Applications: Alkyl halides serve as intermediaries in organic synthesis.
Examples & Applications
Conversion of ethanol to ethyl bromide using PBr3.
Conversion of isopropanol to isopropyl chloride using PCl3.
Memory Aids
Interactive tools to help you remember key concepts
Rhymes
To make a bromide, just take the alcohol, with PBr3, it will answer your call.
Stories
Imagine a party where alcohols dance away and phosphorus trihalides come to replace them with stylish halides.
Memory Tools
Remember A-P-H: Alcohol, Phosphorus, Halide - the sequence of transformation.
Acronyms
APHA
Alcohols Transform to Phosphorus Halides for Alkyls.
Flash Cards
Glossary
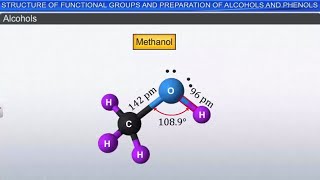
- Alcohol
An organic compound containing one or more hydroxyl (-OH) groups.
- Alkyl Halide
A compound derived from an alkane by replacing one or more hydrogen atoms with halogens.
- Phosphorus Trihalides
Compounds containing phosphorus and three halogen atoms, commonly used as reagents in organic synthesis.
- Alkoxide Ion
An ion formed from an alcohol by the removal of a hydrogen atom from the hydroxyl group.
Reference links
Supplementary resources to enhance your learning experience.
