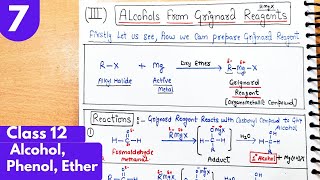
From Grignard reagents
Enroll to start learning
You’ve not yet enrolled in this course. Please enroll for free to listen to audio lessons, classroom podcasts and take practice test.
Interactive Audio Lesson
Listen to a student-teacher conversation explaining the topic in a relatable way.
Introduction to Grignard Reagents
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Today, we're going to discuss Grignard reagents, which are crucial in synthesizing alcohols. Can anyone tell me what a Grignard reagent is?

Isn't it an organomagnesium compound?

Exactly! They're formed by the reaction of alkyl halides with magnesium. Now, can someone explain how these reagents can be utilized in forming alcohols?

They react with carbonyl compounds to produce alcohols, right?

That's correct! This nucleophilic addition forms an alkoxide intermediate, which can be hydrolyzed to yield alcohol. Does anyone remember what type of alcohol forms from methanal?

A primary alcohol!

Good memory! Now, let's recap: Grignard reagents are formed from alkyl halides and magnesium, and they react with carbonyl compounds to make alcohols. This reaction is significant for organic synthesis, as it allows for the creation of diverse alcohols.
The Mechanism of Reaction
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Next, let's delve into the mechanism. Can anyone outline the steps when a Grignard reagent reacts with a carbonyl compound?

First, the Grignard reagent attacks the carbonyl carbon.

Exactly! What happens after that?

It forms a tetrahedral alkoxide.

Right again! And after the formation of this alkoxide, what do we do next?

We hydrolyze it to form the alcohol.

Great job! So the three crucial steps are nucleophilic attack, formation of the alkoxide, followed by hydrolysis. This results in various types of alcohols based on the carbonyl compound used.
Types of Alcohols Produced
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Now, let's examine the types of alcohols produced when using different carbonyl compounds. What will happen with a ketone?

That would yield a tertiary alcohol.

Correct! And what about an aldehyde other than methanal?

It will result in a secondary alcohol.

Now, let's summarize. Grignard reagents not only facilitate alcohol synthesis but also allow us to access different types of alcohols, making them versatile tools for organic chemists.
Introduction & Overview
Read summaries of the section's main ideas at different levels of detail.
Quick Overview
Standard
The section elaborates on how alcohols can be synthesized using Grignard reagents. It details the mechanism of these reactions, including nucleophilic addition and subsequent hydrolysis to yield different classes of alcohols based on the type of carbonyl compound involved.
Detailed
From Grignard Reagents
In the preparation of alcohols, Grignard reagents are key reactants formed from alkyl halides reacting with magnesium metal. When Grignard reagents react with carbonyl compounds such as aldehydes and ketones, they undergo nucleophilic addition to the carbonyl carbon atom. This process generates an alkoxide intermediate that, when hydrolyzed, results in the formation of an alcohol.
The classification of the resulting alcohol—whether primary, secondary, or tertiary—depends on the type of carbonyl compound used. For instance, the reaction of Grignard reagents with methanal yields primary alcohols, while the reaction with other aldehydes produces secondary alcohols, and ketones yield tertiary alcohols. This versatility makes Grignard reagents invaluable in organic synthesis.
Youtube Videos








Audio Book
Dive deep into the subject with an immersive audiobook experience.
Introduction to Grignard Reagents
Chapter 1 of 1
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Alcohols are produced by the reaction of Grignard reagents (Unit 6, Class XII) with aldehydes and ketones. The first step of the reaction is the nucleophilic addition of Grignard reagent to the carbonyl group to form an adduct. Hydrolysis of the adduct yields an alcohol.
Detailed Explanation
When methanal (the simplest aldehyde) reacts with a Grignard reagent, the result is always a primary alcohol. This is because the Grignard reagent adds to the carbon of the carbonyl group, leading to a structure that, when hydrolyzed, provides a primary alcohol. For other aldehydes, the nature of the reaction remains largely the same but leads to secondary alcohols as products. Ketones yield tertiary alcohols in the similar nucleophilic addition reaction. Understanding this mechanism is important as it allows chemists to predict the types of alcohols produced based on the function of the substrate (aldehyde or ketone) being used.
Examples & Analogies
Imagine you are baking a cake. If you start with egg whites (somewhat like methanal), you will end up with a fluffy cake (primary alcohol) when whisked. If instead you use whole eggs (other aldehydes), you'll achieve a denser cake (secondary alcohol). If you use duck eggs (like ketones), the cake becomes very rich (tertiary alcohol) due to the additional fats in the egg.
Key Concepts
-
Grignard Reagents: Essential organomagnesium compounds for alcohol synthesis.
-
Nucleophilic Addition: Key mechanism where nucleophiles react with carbonyls.
-
Classification of Alcohols: Distinction based on the original carbonyl compound.
Examples & Applications
The reaction of methylmagnesium bromide with propanal produces 2-butanol, highlighting the reaction path towards secondary alcohols.
The reaction of phenylmagnesium bromide with acetone yields 2-phenyl-2-propanol, showcasing tertiary alcohol formation.
Memory Aids
Interactive tools to help you remember key concepts
Rhymes
Grignard's bond, a carbon knows, with reactions that bring alcohols to show.
Stories
Imagine a wizard named Grignard who waved his wand over carbonyls, transforming them into magical drinks – the alcohols!
Memory Tools
G-R-A-C-E: Grignard Reagent Adds Carbonyl, Eventually (producing alcohol).
Acronyms
G.R.A.B
Grignard Reacts with Aldehydes and yields a Base.
Flash Cards
Glossary
- Grignard Reagent
A reagent formed by the reaction of an organohalide with magnesium, used in organic synthesis.
- Nucleophilic Addition
A reaction mechanism where a nucleophile forms a bond with a carbon atom in a carbonyl compound.
- Alkoxide Intermediate
A negatively charged species formed by the addition of a nucleophile to a carbonyl compound.
Reference links
Supplementary resources to enhance your learning experience.
