Alpha-particle trajectory
Enroll to start learning
You’ve not yet enrolled in this course. Please enroll for free to listen to audio lessons, classroom podcasts and take practice test.
Interactive Audio Lesson
Listen to a student-teacher conversation explaining the topic in a relatable way.
Introduction to Alpha-Particles and Trajectory
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Today we're discussing alpha-particles and how their trajectories can give us insight into the structure of atoms.

What exactly are alpha-particles?

Excellent question! Alpha-particles are helium nuclei made up of two protons and two neutrons, and they carry a positive charge.

How does the trajectory of these particles relate to atoms?

The trajectory, or path taken by alpha-particles when they are scattered off a nucleus, helps us determine the size and charge distribution within the atom. Specifically, we focus on something called the impact parameter.

What is the impact parameter?

The impact parameter is the perpendicular distance from the alpha-particle's initial velocity vector to the center of the nucleus. It significantly affects how the particle will scatter.

So a smaller impact parameter means a larger scattering angle?

Exactly! A small impact parameter indicates the alpha-particle is closer to the nucleus, which increases the likelihood of a significant scattering angle.

In summary, we discussed alpha-particles and their significance, particularly focusing on the impact parameter and its effect on scattering.
Scattering Experiments and Results
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Let’s discuss the Geiger-Marsden experiment, which used alpha-particles to probe the structure of the atom.

What did the experiment involve?

The experiment directed a beam of alpha-particles at a thin gold foil and measured the scattering angles of the particles that interacted with the nucleus.

What were they trying to find out?

They aimed to determine how concentrated the positive charge—and subsequently the size—of the nucleus was.

What did they discover about the nucleus?

The findings showed that most alpha-particles passed through the foil, but a small fraction were deflected at large angles. This led to the conclusion that the nucleus is small and densely packed.

So this was a crucial step to understand atomic structure?

Precisely! It paved the way for Rutherford's planetary model of the atom, where electrons orbit around a dense nucleus.

To summarize, we talked about the Geiger-Marsden experiment, focusing on the experimental aims and the crucial findings regarding atomic structure.
Interpreting Scattering Results
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Let’s interpret the results of the scattering experiment in detail.

What do the angles of scattering tell us?

Great question! The scattering angles reflect how close the alpha-particles came to the nucleus.

If an alpha-particle scatters at a large angle, does that mean it was near the nucleus?

Exactly! Large angles of scattering imply a close encounter with the nucleus, resulting in significant repulsive forces.

What’s the implication of most particles going straight through?

It suggests that atoms are largely empty space, with most of their mass concentrated in the small nucleus.

So can we estimate the size of the nucleus from this data?

Yes! The patterns of scattering allow us to infer that the nucleus is around 10^-15 meters in size, significantly smaller than the atom itself.

In conclusion, we analyzed how scattering angles inform us about atomic structure, emphasizing the small size and density of the nucleus.
Conclusion on Alpha-Particle Trajectories
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Today, we explored alpha-particle trajectories and their significance in atomic theory. Who can recap the key points?

We learned that the impact parameter affects scattering angles and how a small impact parameter leads to large deflections.

The experiment showed that the nucleus is small and densely packed, leading us to Rutherford's model!

And most of the atom is empty space, which explains why many alpha-particles went right through the foil.

Exactly! The scattering data helped establish a clear picture of atomic structure. Well done, everyone!
Introduction & Overview
Read summaries of the section's main ideas at different levels of detail.
Quick Overview
Standard
This segment presents the trajectory of alpha-particles during collisions with atomic nuclei, emphasizing the role of the impact parameter in the scattering process and the implications for understanding atomic structure, particularly in the development of Rutherford's model of the atom.
Detailed
Alpha-particle Trajectory
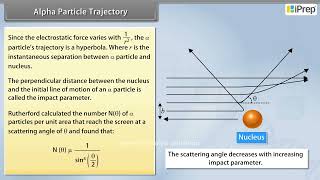
In this section, we explore the trajectory traced by alpha-particles as they interact with atomic nuclei, focusing on the concept of the impact parameter and its significant role in determining the scattering behavior. The impact parameter (b) refers to the perpendicular distance from the initial velocity vector of the alpha-particle to the center of the nucleus. This parameter influences how alpha-particles are scattered in various directions during collisions, revealing essential insights into the atomic structure.
Key Points:
- Impact Parameter Influence: A smaller impact parameter means the alpha-particle is closer to the nucleus, leading to greater scattering angles; conversely, a larger impact parameter results in minimal deflection.
- Scattering Statistics: Most alpha-particles pass through the foil without deflection, indicating that the nucleus is small and densely packed with positive charge. Only a fraction undergoes significant deflection, reinforcing the notion of a concentrated positive nucleus per Rutherford's model.
- Rutherford's Findings: The observations from these trajectories allowed Rutherford to deduce that the atomic mass and positive charge are concentrated in a small nucleus, which profoundly impacted our understanding of atomic structure.
This understanding addresses the complexities of atomic interactions and is foundational for the subsequent models of atomic structure, including the planetary model where electrons orbit a dense nucleus.
Youtube Videos







Audio Book
Dive deep into the subject with an immersive audiobook experience.
Impact Parameter Definition
Chapter 1 of 3
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
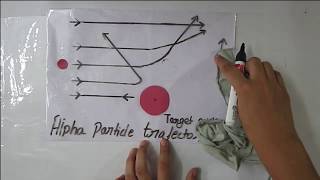
The trajectory traced by an a-particle depends on the impact parameter, b of collision. The impact parameter is the perpendicular distance of the initial velocity vector of the a-particle from the centre of the nucleus.
Detailed Explanation
The impact parameter (b) is a critical concept in understanding how alpha-particles interact with atomic nuclei. It is defined as the shortest distance from the center of the nucleus to the path of the alpha-particle at a right angle. This parameter helps predict the scattering angles: smaller impact parameters mean closer approaches to the nucleus and hence larger scattering angles, while larger impact parameters result in less deflection.
Examples & Analogies
Consider throwing a basketball at a hoop. If you aim directly at the center of the hoop, the ball will go straight in, analogous to a small impact parameter which represents a more direct collision. However, if you throw the ball from the side, it may miss the hoop entirely or just graze it, representing a larger impact parameter and resulting in a smaller chance of a significant interaction.
Scattering Angles and Their Implications
Chapter 2 of 3
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
A given beam of a-particles has a distribution of impact parameters b, so that the beam is scattered in various directions with different probabilities. It is seen that an a-particle close to the nucleus (small impact parameter) suffers large scattering. In case of head-on collision, the impact parameter is minimum and the a-particle rebounds back (q @ p). For a large impact parameter, the a-particle goes nearly undeviated and has a small deflection (q @ 0).
Detailed Explanation
When alpha-particles are directed toward a target nucleus, the scattering angle they undergo after colliding with the nucleus varies according to the impact parameter. If the impact parameter is small, the alpha-particle is more likely to have a significant interaction with the nucleus, leading to a high scattering angle (often referred to as a 'large deflection'). Conversely, if the impact parameter is large, the alpha-particle passes further away and only experiences minor deflection, resembling a glancing blow.
Examples & Analogies
Imagine a game of pool. When the cue ball strikes the eight ball head-on (small impact parameter), it sends the eight ball off at a sharp angle and likely towards a pocket. If the cue ball hits the eight ball from the side (large impact parameter), the eight ball moves away gently, barely changing direction. This similarity illustrates how small and large impact parameters lead to drastically different outcomes in scattering.
Significance of Scattering Data
Chapter 3 of 3
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
The fact that only a small fraction of the number of incident particles rebound back indicates that the number of a-particles undergoing head-on collision is small. This, in turn, implies that the mass and positive charge of the atom is concentrated in a small volume. Rutherford scattering therefore, is a powerful way to determine an upper limit to the size of the nucleus.
Detailed Explanation
The results of the scattering experiment reveal that most alpha-particles pass through the gold foil without significant interaction, while a small fraction bounce back after close encounters with the nucleus. This observation supports the idea that the atomic nucleus, which contains most of the atom's mass and positive charge, is very small compared to the overall size of the atom. Understanding how this scattering works enables scientists to estimate the dimensions of the nucleus accurately.
Examples & Analogies
Think of shooting a BB gun at a dartboard. If most of the BBs pass cleanly through the dartboard (representing the atom) and a few stick to the bullseye (the nucleus), it tells you that the bullseye occupies a tiny proportion of the dartboard's area. Similarly, the scattering data tells us how tiny and dense the nucleus is relative to the rest of the atom, emphasizing the vast amounts of empty space within an atom.
Key Concepts
-
Impact parameter: The perpendicular distance affecting the scattering of particles.
-
Scattering angle: The degree of deflection experienced by an alpha-particle.
-
Rutherford's model: A representation of the atom emphasizing a small nucleus surrounded by electrons.
Examples & Applications
In a scattering experiment, an alpha-particle with a small impact parameter could be deflected back, illustrating strong interactions with the nucleus.
Most alpha-particles passing through gold foil directly indicate that atoms are largely empty space, affirming Rutherford’s model.
Memory Aids
Interactive tools to help you remember key concepts
Rhymes
Small impacts deflect, large ones go straight, particles in orbit, it’s quite a fate.
Stories
Imagine alpha-particles as brave explorers; those with small paths (impact parameters) find immense treasures (deflections) near the nucleus!
Memory Tools
Safely Exploring (SE) means Small angle for Eventful paths near the nucleus.
Acronyms
SIA - Small Impact leads to Angle change in scattering.
Flash Cards
Glossary
- Alphaparticle
A positively charged particle consisting of two protons and two neutrons, essentially a helium nucleus.
- Impact parameter (b)
The perpendicular distance from the initial velocity vector of a particle to the center of a nucleus, influencing scattering outcomes.
- Scattering angle (θ)
The angle at which an alpha-particle is deflected after interacting with a nucleus during scattering.
- Rutherford's planetary model
A model of the atom proposing that a small, dense nucleus is surrounded by orbiting electrons, resembling a solar system.
Reference links
Supplementary resources to enhance your learning experience.
