de BROGLIE'S EXPLANATION OF BOHR'S SECOND POSTULATE OF QUANTISATION
Enroll to start learning
You’ve not yet enrolled in this course. Please enroll for free to listen to audio lessons, classroom podcasts and take practice test.
Interactive Audio Lesson
Listen to a student-teacher conversation explaining the topic in a relatable way.
Introduction to Bohr's Second Postulate
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Today, we’re exploring Bohr's second postulate, which asserts that the angular momentum of an electron orbiting the nucleus is quantised. What do we mean by quantisation?

It means that the angular momentum can only take specific values.

Correct! It can be expressed as L = nh/2π, where n is an integer. Why do you think the angular momentum is restricted in this way?

Maybe because of the characteristics of the electron's movement around the nucleus?

Exactly, and that leads us to the significance of Louis de Broglie's work.
de Broglie's Wave-Particle Duality
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

De Broglie introduced the idea of wave-particle duality. Can anyone explain what this concept means?

It means that particles like electrons exhibit both wave-like and particle-like properties.

Exactly! So how does this relate to Bohr’s model?

I think it shows that the electron's orbit can be thought of as a wave pattern.

Right! De Broglie argued that an electron's circular orbit can form standing waves, crucial for understanding quantisation.
Standing Waves and Quantisation
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

How do we express the condition for these standing waves in an electron's orbit?

The circumference of the orbit must equal an integral number of wavelengths.

Correct! That gives us the equation 2πr = nλ. Can we express λ in terms of momentum?

Yes, λ = h/p, and for electrons, p is mv.

Excellent! Substituting this back gives us the relationship leading to the quantised angular momentum - which is a foundational part of this theory.
Impact of de Broglie's Hypothesis
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

So why is de Broglie's hypothesis significant in the context of atomic theory?

It provides a framework for understanding how electrons behave in atoms.

Yes! It connects classical and modern physics, emphasizing the necessity for wave mechanics in explaining atomic behavior.

Does this mean modern theories have evolved from this idea?

Precisely! It paved the way for advancements in quantum mechanics.
Introduction & Overview
Read summaries of the section's main ideas at different levels of detail.
Quick Overview
Standard
This section explores the explanation provided by Louis de Broglie for Bohr's second postulate, which states that the angular momentum of an electron in a stable orbit is quantised. De Broglie introduced the notion of wave-particle duality for electrons, proposing that their circular motion can be understood through the concept of standing waves.
Detailed
Detailed Summary
In this section, we delve into de Broglie's pivotal contributions to atomic theory, particularly focusing on his explanation of Bohr's second postulate regarding the quantisation of angular momentum. According to Bohr, the angular momentum of an orbiting electron is restricted to integral multiples of
h/2π, a concept that seems puzzling without a clear rationale.
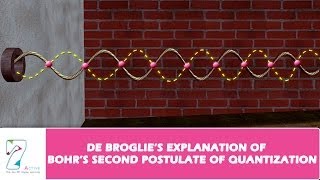
De Broglie addressed this ambiguity by describing electrons as having wave properties. He suggested that, akin to waves on a string that resonate in stationary patterns, electrons in their orbits must also create standing waves. This condition necessitates that the circumference of the electron's orbit equals an integral number of de Broglie wavelengths:
2πr = nλ, where λ is the wavelength of the electron given by λ = h/p (with p being momentum). Therefore, relating the wavelength to the momentum p = mv led to the quantisation condition: mv r = n h/2π, which mirrors Bohr's postulate.
This finding links wave properties to particles, showing that quantised electron orbits stem from the wave nature of electrons, thus reinforcing the standing wave concept in atomic structure. De Broglie's hypothesis not only clarified quantum mechanics but also provided a foundational basis for further developments in quantum theory.
Youtube Videos








Audio Book
Dive deep into the subject with an immersive audiobook experience.
Bohr's Second Postulate Overview
Chapter 1 of 6
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Of all the postulates, Bohr made in his model of the atom, perhaps the most puzzling is his second postulate. It states that the angular momentum of the electron orbiting around the nucleus is quantised (that is, L = nh/2p; n = 1, 2, 3 …). Why should the angular momentum have only those values that are integral multiples of h/2p?
Detailed Explanation
This chunk introduces Bohr's second postulate, which states that the angular momentum (L) of an electron in orbit is quantized. This means that L can only take on certain discrete values, which are multiples of the Planck constant divided by 2π (h/2π). The question posed highlights the intriguing nature of this idea, as it suggests that only specific states of motion are allowed for the electron, making the concept of quantization essential for understanding atomic behavior.
Examples & Analogies
Think of a musician tuning a guitar. The musician can only play notes that correspond to specific frequencies, which are determined by the lengths of the guitar strings. Similarly, just as only certain musical notes (or frequencies) can resonate on the guitar, electrons can only occupy specific orbits characterized by quantized angular momentum.
De Broglie's Contribution
Chapter 2 of 6
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
The French physicist Louis de Broglie explained this puzzle in 1923, ten years after Bohr proposed his model. We studied, in Chapter 11, about the de Broglie’s hypothesis that material particles, such as electrons, also have a wave nature. C. J. Davisson and L. H. Germer later experimentally verified the wave nature of electrons in 1927.
Detailed Explanation
This chunk focuses on Louis de Broglie's contribution, which provides a crucial evolution of Bohr's model. De Broglie suggested that electrons exhibit wave-like properties and that these waves can form standing waves in specific conditions. By recognizing that particles can behave like waves, he helped explain why the angular momentum of the electron must be quantized; only certain wavelengths or standing wave patterns would fit around the nucleus without interference.
Examples & Analogies
Imagine a piece of rope being shaken. If you shake it at specific frequencies, you can create standing waves with consistent patterns. If you try to shake it at random frequencies, you won't produce clear wave patterns. Electrons work similarly; they can only exist in specific wave patterns around the nucleus, leading to the quantization of their angular momentum.
Standing Waves and Orbits
Chapter 3 of 6
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Louis de Broglie argued that the electron in its circular orbit, as proposed by Bohr, must be seen as a particle wave. In analogy to waves travelling on a string, particle waves too can lead to standing waves under resonant conditions. For an electron moving in nth circular orbit of radius r, the total distance is the circumference of the orbit, n, 2πr.
Detailed Explanation
In this chunk, de Broglie's perspective emphasizes the wave nature of electrons. When an electron travels in a circular orbit, it can be thought of as a wave whose path creates a loop. The circumference of the orbit must equal an integral number of wavelengths for constructive interference, allowing only certain orbits to exist. This condition is crucial for establishing the relationship between the wave characteristics of electrons and their quantized energy levels.
Examples & Analogies
Consider playing a roundabout merry-go-round at just the right speed; if you spin at the right speed (like the wavelength matching the circumference), everyone stays stable without falling off. If you go too fast or too slow, it becomes chaotic, similar to how electrons can only remain stable in specific, quantized paths in their atomic orbits.
Quantization of Angular Momentum
Chapter 4 of 6
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Thus, from Eq. (12.12), we have 2πr = nλ, where λ is the de Broglie wavelength of the electron moving in nth orbit. If the speed of the electron is much less than the speed of light, the momentum is mv. Thus, λ = h/p. Then we have mvr = nh/2π. This is the quantum condition proposed by Bohr for the angular momentum of the electron.
Detailed Explanation
Here, we see the culmination of de Broglie's hypothesis leading back to Bohr's condition for quantized angular momentum. By establishing a relationship between the electron's wavelength and its orbital path, de Broglie proved that the quantization of angular momentum arises naturally from the wave nature of electrons. When combined with Bohr's concept, it reinforces the conditions under which electrons can exist in stable orbits.
Examples & Analogies
Think about a jumping dancer. The dancer’s position can represent different energy states. They can only stay on specific spots (like the quantized orbits) where everything is in sync (standing waves). If they try to jump to an in-between spot (not a multiple of their basic rhythm), they’re likely to lose balance and fall off completely, much like how electrons can only occupy certain orbits and not positions in between.
Implications of De Broglie's Hypothesis
Chapter 5 of 6
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Thus de Broglie hypothesis provided an explanation for Bohr’s second postulate for the quantisation of angular momentum of the orbiting electron. The quantised electron orbits and energy states are due to the wave nature of the electron and only resonant standing waves can persist.
Detailed Explanation
This chunk emphasizes the significance of de Broglie's hypothesis in explaining the quantization of the electron orbits as proposed by Bohr. By recognizing that electrons behave as waves, de Broglie illustrated that only certain resonant wave patterns are sustainable around the nucleus, allowing for stable orbits. This understanding was fundamental in advancing the field of quantum mechanics and further refining atomic models.
Examples & Analogies
Consider how a choir sings in harmony. Only certain combinations of notes create beautiful music, while random pitches create discord. Similarly, the electrons that manage to exist as resonant standing waves create stable ‘music’ for the atom, while any attempt to exist outside those specific states leads to instability.
Limitations of the Bohr Model
Chapter 6 of 6
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Bohr’s model, involving classical trajectory picture (planet-like electron orbiting the nucleus), correctly predicts the gross features of the hydrogenic atoms but cannot be generalized to complex atoms. Some limitations include: (i) The Bohr model is applicable to hydrogenic atoms. It cannot be extended to even two electron atoms such as helium. (ii) While the Bohr’s model correctly predicts the frequencies of the light emitted by hydrogenic atoms, the model is unable to explain the relative intensities of the frequencies in the spectrum.
Detailed Explanation
In this concluding chunk, the limitations of Bohr's model are outlined. While it successfully describes the electron behaviors in hydrogen (one electron), it fails with more complex atoms. The interactions between multiple electrons and how they influence each other lead to difficulties in predictions. Thus, while Bohr’s model was a significant step forward, it serves as a foundation rather than a final explanation for atomic structure.
Examples & Analogies
Think of a simple recipe for a dish that only uses a few ingredients. It can be simple and specific. But if you add more ingredients or layer them differently, the recipe starts to fall apart. Similarly, Bohr's model works well for hydrogen but can't accommodate the complex ‘recipes’ of multi-electron atoms.
Key Concepts
-
de Broglie's Hypothesis: Suggests that electrons have wave-like properties.
-
Quantisation of Angular Momentum: States that angular momentum is quantised as L = nh/2π.
-
Standing Waves: Formed in the electron's orbit, leading to the quantisation condition.
-
Wave-Particle Duality: Refers to the dual nature of matter, where particles exhibit wave-like behavior.
Examples & Applications
When an electron travels in a circular orbit around the nucleus, its wave-like nature leads to the formation of standing waves, which results in quantised angular momentum.
De Broglie's de Broglie wavelength can be used to calculate the wavelength associated with an electron moving in an atomic orbit, demonstrating the quantum nature of particles.
Memory Aids
Interactive tools to help you remember key concepts
Rhymes
Angular momentum is a fixed play, h over two pi, just a few ways.
Stories
Imagine a dancer in a circle, moving around the stage. She spins and twirls, but her steps must fit into defined patterns—much like how electrons must fit into specific orbits in an atom.
Memory Tools
For wave-particle duality: 'Waves are particles, P's and W's; they both exist, swap their shoes.'
Acronyms
Remember 'WAVE' for Wave-particle duality
- Wave nature
- Angular momentum
- Velocity
- Electron.
Flash Cards
Glossary
- Quantisation
The process of constraining a certain quantity, such as angular momentum, to discrete values rather than continuous ones.
- WaveParticle Duality
The concept that all particles exhibit both wave-like and particle-like properties.
- Standing Waves
Waves that remain in a constant position, formed from the interference of two waves traveling in opposite directions.
- de Broglie Wavelength
The wavelength associated with a particle, defined as λ = h/p, where h is Planck's constant and p is momentum.
- Angular Momentum
A measure of the amount of rotational motion an object has, calculated as L = mv r.
Reference links
Supplementary resources to enhance your learning experience.
