Activity 11.4
Enroll to start learning
You’ve not yet enrolled in this course. Please enroll for free to listen to audio lessons, classroom podcasts and take practice test.
Interactive Audio Lesson
Listen to a student-teacher conversation explaining the topic in a relatable way.
Conductivity of Liquids
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Today, we're going to explore whether distilled water conducts electricity. Who can remind me what a good conductor is?

A good conductor is a material that allows electric current to pass through easily.

Exactly! So what do you think will happen when we test distilled water?

I think it might not glow because it doesn't have enough ions.

Great prediction! Let's test it. Remember, distilled water is pure and lacks ions. So can we classify it as a good or poor conductor?

It's a poor conductor!

Correct! Let's check it using our tester.

The bulb didn't glow. So distilled water really is a poor conductor!

Exactly! Now, what about if we add salt to the distilled water? What do you think will happen?

I think it will conduct electricity because salt has ions.

Let’s find out!
Testing Salt Solutions
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Now that we have added salt to our distilled water, let's retest our solution. What do you expect to see with the tester?

I believe the bulb will glow this time!

Yes, because salt dissociates into ions in water, allowing electricity to flow. Let's see!

Wow! The bulb glows brightly!

That’s right! This shows that the salt solution is now a good conductor of electricity. Can someone tell me why this happens?

Because the salt dissolves and breaks into charged particles!

Excellent explanation! Remember, good conductors have freely moving ions, which is what we need for the electricity to flow.
Chemical Effects of Electric Current
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Now that we know salt solutions conduct electricity, can anyone tell me what happens when electricity passes through these solutions?

It could cause chemical reactions.

Correct! When electric current passes through a conducting solution, it can cause changes, like producing gases or depositing metals. What kind of gas might we see?

Maybe bubbles from oxygen or hydrogen?

That's right! We can observe bubbles forming on the electrodes. These reactions are part of the chemical effects of electric current.

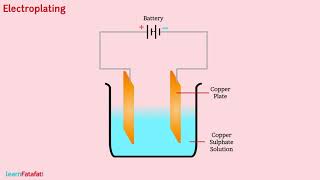
So, is this how electroplating works too?

Good connection! Yes, in electroplating, a layer of metal is deposited onto an object through the same principles we’ve discussed today.
Introduction & Overview
Read summaries of the section's main ideas at different levels of detail.
Quick Overview
Standard
In this section, students investigate whether distilled water conducts electricity, finding that it does not until salt is added. The section introduces the concept of good and poor conductors and describes the chemical effects of electric current in various solutions.
Detailed
In-Depth Summary of Activity 11.4
In Activity 11.4, students test whether distilled water can conduct electricity and observe that it does not. However, upon dissolving a pinch of salt in it, the water becomes conductive, demonstrating that the presence of ions enhances conductivity. This highlights the distinction between good and poor conductors of electricity, notably that distilled water is a poor conductor due to its lack of dissolved minerals, while saltwater acts as a good conductor due to the ionic dissociation of salt in water. Furthermore, the section emphasizes the significance of understanding how electric current interacts with different materials, leading to chemical reactions that can produce gases and deposits of metals when direct current passes through conductive solutions.
Youtube Videos







Audio Book
Dive deep into the subject with an immersive audiobook experience.
Testing Distilled Water
Chapter 1 of 2
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Take about two teaspoonfuls of distilled water in a clean and dry plastic or rubber cap of a bottle. (You may obtain distilled water from your school science lab. You may also get distilled water from a medical store or a doctor or a nurse) . Use the tester to test whether distilled water conducts electricity or not. What do you find? Does distilled water conduct electricity? Now dissolve a pinch of common salt in distilled water. Again test. What do you conclude this time?
Detailed Explanation
In this chunk, the activity involves testing distilled water to see if it conducts electricity. The initial observation is to check if pure distilled water can carry an electric current. Typically, distilled water is a poor conductor of electricity because it lacks dissolved minerals or salts that facilitate conduction. Once salt is added, it dissociates into ions that can move freely in water, thus testing the solution again will show that it can now conduct electricity effectively. This demonstrates the difference between distilled water (which doesn't conduct well) and salted water (which does).
Examples & Analogies
Think of distilled water like a quiet, still pond where very few fish can be seen. When you add salt, it's like feeding the pond's fish, causing them to swim around energetically. The movement of these fish represents how ions create a pathway for electricity to flow.
Conclusion on Conductivity
Chapter 2 of 2
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
When salt is dissolved in distilled water, we obtain salt solution. This is a conductor of electricity. The water that we get from sources such as taps, hand pumps, wells, and ponds is not pure. It may contain several salts dissolved in it. Small amounts of mineral salts are naturally present in it. This water is thus a good conductor of electricity. On the other hand, distilled water is free of salts and is a poor conductor. Most liquids that conduct electricity are solutions of acids, bases, and salts.
Detailed Explanation
This segment further explains the findings regarding distilled water and how it relates to conductivity. It emphasizes that distilled water, being free from dissolved salts, does not conduct electricity well. Conversely, natural water supplies typically contain these salts and thus serve as better conductors. The mention of acids and bases highlights that many substances have the ability to conduct electricity because they break down into ions in solution. This is key in understanding how various solutions behave differently in terms of electric current.
Examples & Analogies
Imagine a crowded highway (tap water) where many cars (ions) can easily move and overtake each other, allowing things to flow smoothly. In contrast, a narrow country road (distilled water) lacks vehicles (ions), making it difficult for anything to pass through.
Key Concepts
-
Conductors and Insulators: Understanding the difference between materials that conduct electricity well and those that do not.
-
Chemical Effects: Recognizing that electric current can cause chemical reactions in conductive solutions.
-
Role of Ions: Identifying how dissolved substances like salt produce ions, making solutions good conductors.
Examples & Applications
Saltwater is a good conductor because it contains ions from the dissolved salt.
Distilled water is a poor conductor as it lacks dissolved minerals and ions necessary for conducting electricity.
Memory Aids
Interactive tools to help you remember key concepts
Rhymes
Salt dissolves, ions dance, Electricity flows, giving chance.
Stories
Imagine distilled water as a locked door, no one can get through. But when salt arrives, it opens and lets the electricity flow freely!
Memory Tools
SALT - Solutions Allow Lights to Glow.
Acronyms
ICE - Ions Conduct Electricity.
Flash Cards
Glossary
- Good Conductor
A material that allows electric current to pass through easily.
- Poor Conductor
A material that does not allow electric current to pass through easily.
- Ions
Charged particles that are formed when a compound, such as salt, dissolves in water.
- Electrolyte
A substance that produces an electrically conducting solution when dissolved in a polar solvent, like water.
- Electroplating
The process of depositing a layer of a desired metal on another material using electric current.
Reference links
Supplementary resources to enhance your learning experience.
