What You Have Learnt
Enroll to start learning
You’ve not yet enrolled in this course. Please enroll for free to listen to audio lessons, classroom podcasts and take practice test.
Interactive Audio Lesson
Listen to a student-teacher conversation explaining the topic in a relatable way.
Conductivity of Liquids
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Today we're going to learn about how different liquids conduct electricity. Can anyone tell me what they think makes a liquid a good conductor?

Maybe it depends on what the liquid is made of?

Exactly! Liquids that have ions are usually good conductors. For instance, lemon juice conducts electricity because it contains citric acid which dissociates into ions.

What about distilled water? Does it conduct electricity?

Great question! Distilled water is actually a poor conductor because it has very few ions. However, if we add salt to it, it allows electricity to flow more easily.

Can we test any liquid to see if it conducts?

Yes, we can! We'll be creating a simple tester to check different liquids today. Remember, conductors can complete the circuit and light up our bulb!

In summary, liquids conduct electricity depending on their ion content: good conductors have high ion concentration.
Chemical Reactions from Electric Current
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

When we pass electric current through a liquid, what do you think happens?

Is there a chemical change?

Exactly! We observe gas bubbles forming and sometimes even color changes in the solution. This is known as the chemical effect of electric current.

So what exactly is happening at the electrodes when we see these bubbles?

The electric current can cause ions to move, resulting in various reactions. For example, oxygen will form at the positive electrode and hydrogen at the negative electrode during electrolysis.

Can you remind us what the term for this process is?

Sure! This process is called electrolysis. It's an important concept in understanding how electric current can influence chemical reactions.

To summarize, the chemical effects caused by electric current lead to gas formation and possible changes in the solution's color.
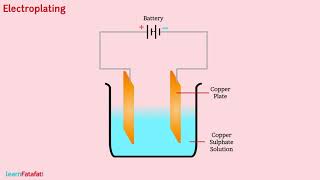
Introduction to Electroplating
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Now, let's discuss electroplating. Does anyone know what electroplating is?

Is it like covering one metal with another?

Yes! In electroplating, we deposit a layer of one metal onto another. This is done using the concept of electrolysis.

What is the purpose of electroplating?

Electroplating is often used to enhance the appearance of objects, prevent corrosion, and make them more durable. For example, bike parts or jewelry are commonly electroplated with a layer of gold or silver.

Can we try a simple electroplating experiment?

Certainly! We'll set up an experiment using copper sulfate and copper plates to see the principle of electroplating in action.

In summary, electroplating uses the electric current to deposit metal and has practical applications in everyday life.
Introduction & Overview
Read summaries of the section's main ideas at different levels of detail.
Quick Overview
Standard
The section elaborates on whether liquids can conduct electricity, detailing the experiments conducted with various substances. It explains the chemical reactions that occur when electric current passes through conducting liquids and the principle of electroplating as an application of these concepts.
Detailed
In this section, we explore the chemical effects of electric current, addressing the conductivity of various liquids such as lemon juice and distilled water. Through several activities, readers are guided to test the conductivity of different substances using a homemade tester, which lights up when electricity flows, indicating conduction. It introduces the idea that while some liquids are good conductors, others are not, and explains that the conductivity depends on the presence of ions in these solutions. The text also discusses electroplating, a process that utilizes the principles of electrolysis to deposit a layer of metal onto another substance, demonstrating practical applications of chemical effects of electric current.
Youtube Videos









Audio Book
Dive deep into the subject with an immersive audiobook experience.
Conductivity of Liquids
Chapter 1 of 3
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
ÜSome liquids are good conductors of electricity and some are poor conductors. ÜMost liquids that conduct electricity are solutions of acids, bases and salts.
Detailed Explanation
This chunk describes how different liquids behave when it comes to conducting electricity. Some liquids allow electric current to pass through easily, while others do not. Good conductors include solutions that contain acids, bases, or salts, which can break down into charged particles that can move freely and carry an electric current.
Examples & Analogies
Think of electricity flowing like water in a pipe. If the pipe is wide and clear (like saltwater), water flows easily. But if the pipe is narrow and clogged (like distilled water), water cannot flow well. This helps us understand why some liquids are good conductors while others are not.
Chemical Effects of Electric Current
Chapter 2 of 3
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
ÜThe passage of an electric current through a conducting liquid causes chemical reactions. The resulting effects are called chemical effects of currents.
Detailed Explanation
When electricity passes through a conducting liquid, it can cause changes in that liquid. These changes might include producing gases, forming new substances, or changing the color of the liquid. This happens because the electric current triggers chemical reactions between substances present in the liquid.
Examples & Analogies
Imagine a magician performing a trick. When a certain spell is cast (the electric current), unexpected things happen—like a color change or bubbles forming—representing the chemical reactions caused by the electric current.
Electroplating Process
Chapter 3 of 3
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
ÜThe process of depositing a layer of any desired metal on another material, by means of electricity, is called electroplating.
Detailed Explanation
Electroplating involves using electricity to deposit a thin layer of metal onto another material, such as coating a cheaper metal with a more valuable one. This is done by immersing two electrodes in a solution containing the metal ions, allowing the current to flow, which attracts the metal ions to the electrode and plates them on its surface.
Examples & Analogies
Think about how a delicious chocolate coating can make a simple biscuit more appealing. In the same way, electroplating improves the appearance and properties of metal objects, making them shiny, less reactive, or more durable.
Key Concepts
-
Conductivity of Liquids: The ability of a liquid to allow electric current to pass through.
-
Electrolysis: The process of using electric current to drive a chemical reaction.
-
Electroplating: A technique used to deposit a layer of metal onto another object using electricity.
Examples & Applications
Lemon juice can conduct electricity because it contains ions.
Electroplating is used to make jewelry appear more presentable.
Memory Aids
Interactive tools to help you remember key concepts
Rhymes
Electrons flow, ions show, liquids that can let it go!
Stories
A curious scientist wonders about different liquids. He tests lemon juice and distilled water. To his surprise, lemon juice lights up the bulb because it has ions while distilled water doesn’t.
Memory Tools
Good Conductors Are Lively—G, C, A, L (Good for metals like Gold, Copper, Aluminum, Lemon Juice).
Acronyms
ELECTRO - Electric currents Lead to Electrolysis for Creating Truly Reliable Objects.
Flash Cards
Glossary
- Electrode
A conductor through which electricity enters or leaves an electrolyte cell.
- Electroplating
The process of coating an object with a layer of metal using electrical current.
- Good Conductor
A material or liquid that allows the flow of electric current easily.
- LED
A type of semiconductor light source that emits light when current flows through it.
- Poor Conductor
A material or liquid that does not allow electric current to flow easily.
Reference links
Supplementary resources to enhance your learning experience.
