Activity 11.7
Enroll to start learning
You’ve not yet enrolled in this course. Please enroll for free to listen to audio lessons, classroom podcasts and take practice test.
Interactive Audio Lesson
Listen to a student-teacher conversation explaining the topic in a relatable way.
Introduction to Electroplating
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Today we are going to learn about electroplating. Can anyone tell me what electroplating is?

Isn't it when one metal is coated with another metal using electricity?

Exactly! It allows us to transfer a metal onto another surface. Can you think of where we might see this in everyday life?

Maybe in jewelry where gold is used to coat cheaper metals?

Great example! Another common use is in car parts where chrome plating makes them shiny and corrosion-resistant. Remember, we use 'Electroplating' to enhance the quality and appearance of less expensive items.
Understanding the Experiment
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Let's set up our experiment for electroplating. What materials do we need?

We need copper sulfate solution and copper plates.

Correct! After dissolving the copper sulfate in distilled water, how do we connect our setup to observe electroplating?

We connect the copper plates to a battery and put them in the copper sulfate solution.

Right! The copper ions will move towards the negative plate, where they will get deposited. This shows how electric current causes chemical changes—let's call this the **'current cause'** mnemonic!
Observations from the Experiment
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

What changes do we expect to see after running our electroplating experiment?

We should see some copper built up on the negative plate.

Yes! And what about the solution itself?

I think some copper will be lost from the solution if it deposits on the plate!

Exactly! The solution will regenerate as the copper ions are transferred. This is important because it highlights that electroplating doesn't waste materials!
Environmental Concerns
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

While electroplating is useful, it poses environmental challenges. Can anyone guess what these might be?

Maybe the chemicals used in the process could be harmful?

Exactly! Proper disposal is crucial. We can't just dump leftover solutions; they require careful handling. Let's remember the acronym **'C.E.D'** for Careful Electroplating Disposal.

So, we have to think about how our actions impact the environment too!
Introduction & Overview
Read summaries of the section's main ideas at different levels of detail.
Quick Overview
Standard
In this section, the principles of electroplating are outlined, illustrating how electric current can be used to transfer metals from one electrode to another. The significance of conducting solutions and the role of various electrodes is also discussed.
Detailed
Detailed Summary
In this section, we discuss electroplating, which is the process of depositing a layer of one metal onto another using electric current. Initially, we prepare copper sulfate solution in a beaker for the experiment. By connecting copper plates to a battery and immersing them into the copper sulfate solution, we can observe the transfer of copper ions from one electrode to another. The electrode connected to the negative terminal attracts the copper ions, leading to the deposition of copper. This process not only demonstrates the principle of electroplating but also highlights the regenerative aspect of the copper sulfate solution, where an equal amount of copper gets dissolved from the other electrode to maintain balance.
Electroplating has significant applications in industries, allowing for the coating of cheaper metals with more expensive metals to enhance their appearance and durability. Safety and environmental concerns are also addressed in the section, urging careful disposal of chemical solutions used in electroplating.
Youtube Videos









Audio Book
Dive deep into the subject with an immersive audiobook experience.
Overview of Electroplating Activity
Chapter 1 of 3
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
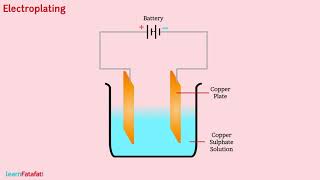
We will need copper sulphate and two copper plates of size around 10 cm × 4 cm. Take 250 mL of distilled water in a clean and dry beaker. Dissolve two teaspoonfuls of copper sulphate in it. Add a few drops of dilute sulphuric acid to copper sulphate solution to make it more conducting. Clean copper plates with sand paper. Now rinse them with water and dry them. Connect the copper plates to the terminals of a battery and immerse them in copper sulphate solution.
Detailed Explanation
In this activity, you are preparing to demonstrate the process of electroplating, which involves depositing a layer of metal onto another object using electricity. To start, you need to create a solution by mixing copper sulphate in distilled water; this setup acts as a conductor of electricity when connected to a battery. The two cleaned copper plates will serve as electrodes, where one is connected to the positive terminal (anode) and the other to the negative terminal (cathode).
Examples & Analogies
Think of electroplating like putting a shiny armor on a common warrior (the base metal). Just as a warrior may not be as impressive without armor, the base metal may not be attractive. By using electricity to ‘wear’ an outer layer of copper, the appearance is greatly enhanced without changing the base metal itself.
Process of Electroplating Explained
Chapter 2 of 3
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
When electric current is passed through the copper sulphate solution, copper sulphate dissociates into copper and sulphate. The free copper gets drawn to the electrode connected to the negative terminal of the battery and gets deposited on it. But what about the loss of copper from the solution? From the other electrode, a copper plate, an equal amount of copper gets dissolved in the solution. Thus, the loss of copper from the solution is restored and the process continues.
Detailed Explanation
During electroplating, an electric current causes the copper sulphate solution to break down into its components: copper ions and sulphate ions. The copper ions move towards the negatively charged electrode (cathode), where they gain electrons and are deposited as solid copper metal. Simultaneously, the copper from the positively charged electrode (anode) dissolves into the solution to maintain balance. This creates a continuous cycle that allows the electroplating to happen without depleting the solution.
Examples & Analogies
Imagine this process as a dance: while some dancers (copper ions) join the main performance (the cathode), other dancers (copper metal from the anode) leave to allow for more room. This cycle keeps the dance floor full — or in this case, keeps the solution balanced with copper.
Interchanging Electrodes in Electroplating
Chapter 3 of 3
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
After doing the electroplating activity, Paheli interchanged the electrodes and repeated the activity. What do you think she would observe this time?
Detailed Explanation
By switching the connections of the electrodes, the roles of the electrodes are reversed: what was previously the cathode becomes the anode and vice versa. This means that if Paheli now passes the current again, instead of copper being deposited on the previously negative electrode, it will be deposited on the previously positive electrode. This demonstrates that electroplating can be affected by the direction of the current flow and the connections made in the circuit.
Examples & Analogies
Imagine switching roles in a classroom where a teacher (anode) usually instructs students and now becomes a student (cathode) following the teacher’s orders. This switch would result in a completely different dynamic and outcome in the learning environment, just as it does in the electroplating process.
Key Concepts
-
Electroplating: A method to apply a coating of metal onto another surface via electric current.
-
Electrodes: The two conductive parts connected to the battery, where the chemical reactions occur.
-
Copper Sulfate Solution: Used in electroplating as a source of copper ions.
Examples & Applications
Jewelry is often electroplated with gold to improve appearance.
Tin cans are electroplated with tin to protect the iron from rusting.
Memory Aids
Interactive tools to help you remember key concepts
Rhymes
Electroplate, don't hesitate! Metal's fate, to decorate!
Stories
A jeweler wanted to shiny up his silver ring. With a sprinkle of copper sulfate and some electric zing, his ring glimmered delightfully – electroplating in action!
Memory Tools
C.E.D: Careful Electroplating Disposal for environmental safety!
Acronyms
ICE
Ions Conduct Electricity!
Flash Cards
Glossary
- Electrode
A conductor through which electric current enters or leaves a medium in an electrochemical cell.
- Electroplating
The process of depositing a layer of one metal onto another using electrical current.
- Good Conductor
Materials that allow electric current to flow easily.
- LED
Light Emitting Diode, a semiconductor light source that emits light when current flows through it.
- Poor Conductor
Materials that do not allow electric current to flow easily.
Reference links
Supplementary resources to enhance your learning experience.
