Chemical Effects of Electric Current - 11.2
Enroll to start learning
You’ve not yet enrolled in this course. Please enroll for free to listen to audio lessons, classroom podcasts and take practice test.
Interactive Audio Lesson
Listen to a student-teacher conversation explaining the topic in a relatable way.
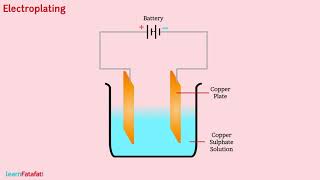
Electroplating and Applications
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Lastly, let's discuss electroplating. Who can tell me what this process involves?

It's when a layer of one metal is deposited over another using electricity!

Exactly! Can anyone give an example of where we see electroplating in real life?

Jewelry often uses electroplating to coat cheaper metals with gold!

Perfect! Remember the acronym EP for Electroplating Process — it’s crucial for many industries.
Introduction & Overview
Read summaries of the section's main ideas at different levels of detail.
Quick Overview
Standard
In this section, we investigate the chemical effects of electric current on various substances, particularly liquids. Through engaging experiments, we test different solutions to determine their conductive properties and observe the resulting chemical changes, including electroplating. The section emphasizes the importance of understanding electricity's role in chemical interactions.
Detailed
The section discusses the chemical effects of electric current, demonstrating that certain liquids act as conductors of electricity and can undergo chemical reactions when current passes through them. This is explored through a series of experiments involving a tester circuit and various liquids, such as lemon juice and distilled water, showing differences between good and poor conductors. The text also addresses the concept of electroplating, explaining how it deposits one metallic layer on another using electric current, providing real-world applications in various industries. The section concludes with a summary of key concepts, emphasizing the relationship between electric current and chemical changes.
Youtube Videos








Audio Book
Dive deep into the subject with an immersive audiobook experience.
Introduction to Conductivity
Chapter 1 of 5
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Your elders might have cautioned you against touching an electrical appliance with wet hands. But do you know why it is dangerous to touch an electrical appliance with wet hands? We have learned earlier that materials, which allow electric current to pass through them, are good conductors of electricity. On the other hand, materials, which do not allow electric current to pass through them easily, are poor conductors of electricity.
Detailed Explanation
Electric current can flow through certain materials more easily than others. Good conductors, such as metals, allow electricity to flow freely. Poor conductors, like rubber and plastic, resist the flow of electricity. When wet hands touch an electrical appliance, the moisture makes the skin a better conductor, increasing the risk of electric shock.
Examples & Analogies
Imagine trying to slide down a smooth slide (good conductor) versus a rough one (poor conductor). The smooth slide allows you to move quickly and easily, just like electricity moves through good conductors. The rough slide slows you down, similar to how electric current struggles through poor conductors.
Testing Liquids for Conductivity
Chapter 2 of 5
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
To test whether a liquid allows electric current to pass through it or not, we can use the same tester. However, replace the cell by a battery. Also, before using the tester we should check whether it is working or not.
Detailed Explanation
You can use a simple circuit tester, made with a battery and a bulb, to test if a liquid conducts electricity. By dipping the tester's ends into the liquid, you can see if the bulb lights up. If it does, the liquid conducts electricity; if not, it does not. This is a crucial step in determining the conductive properties of different liquids.
Examples & Analogies
Think of the tester like a light switch. If you flip the switch and the light turns on, energy is flowing through the wires. Similarly, if the bulb lights up when you dip the tester into a liquid, it means that the electric current is flowing through that liquid.
Conductivity of Common Liquids
Chapter 3 of 5
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Collect a few small plastic or rubber caps of discarded bottles and clean them. Pour one teaspoon of lemon juice or vinegar in one cap. Bring your tester over this cap and let the ends of the tester dip into lemon juice or vinegar. Does the bulb of the tester glow? Does lemon juice or vinegar conduct electricity?
Detailed Explanation
When testing lemon juice or vinegar with a tester, you observe whether the bulb lights up. If the liquid conducts electricity, this means it contains ions that facilitate the flow of current, indicating it is a good conductor. On the other hand, if there is no light, the liquid is a poor conductor.
Examples & Analogies
This can be likened to how roads might allow traffic to pass smoothly during clear weather (good conductor) but become congested and slow when it rains or there are obstacles (poor conductor). Just as traffic needs clear roads, electric current needs conductive materials to flow.
Electrolysis and Chemical Changes
Chapter 4 of 5
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
When electric current flows through a conducting solution, it produces an effect on the solution. The passage of an electric current through a conducting solution causes chemical reactions. As a result, bubbles of a gas may be formed on the electrodes.
Detailed Explanation
Electrolysis involves sending an electric current through a solution, leading to chemical reactions that can produce gas bubbles and sometimes change the color of the solution. For instance, oxygen bubbles may form at one electrode while hydrogen bubbles form at the other, demonstrating the chemical effects of electricity in action.
Examples & Analogies
You can think of this process like baking bread. When you mix the ingredients and apply heat, a chemical reaction occurs that transforms the dough into a fluffy loaf. Similarly, passing electric current through a solution causes alterations at the molecular level, resulting in gases or new materials forming.
Conclusion and Safety Precautions
Chapter 5 of 5
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Small amounts of mineral salts present naturally in water are beneficial for human health. However, these salts make water a good conductor. So, we should never handle electrical appliances with wet hands or while standing on a wet floor.
Detailed Explanation
While some salts in water are good for health, they also enhance the water's conductivity, increasing the risk of electric shock if someone is handling electrical equipment. Thus, safety measures are essential when working around electrical devices and liquids.
Examples & Analogies
Imagine walking on a slippery floor after mopping. Wet surfaces can cause you to slip and fall; similarly, wet hands increase the danger of electric shock due to enhanced conductivity. Always be cautious to avoid combining electricity with water.
